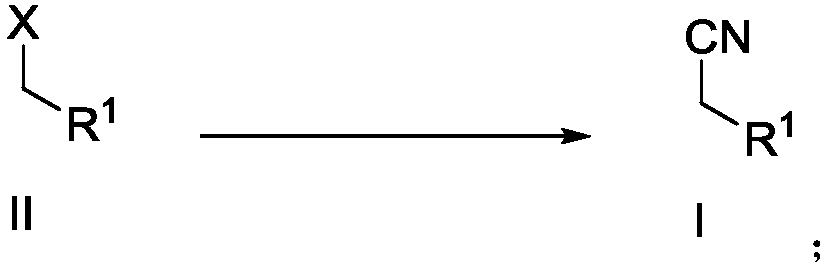
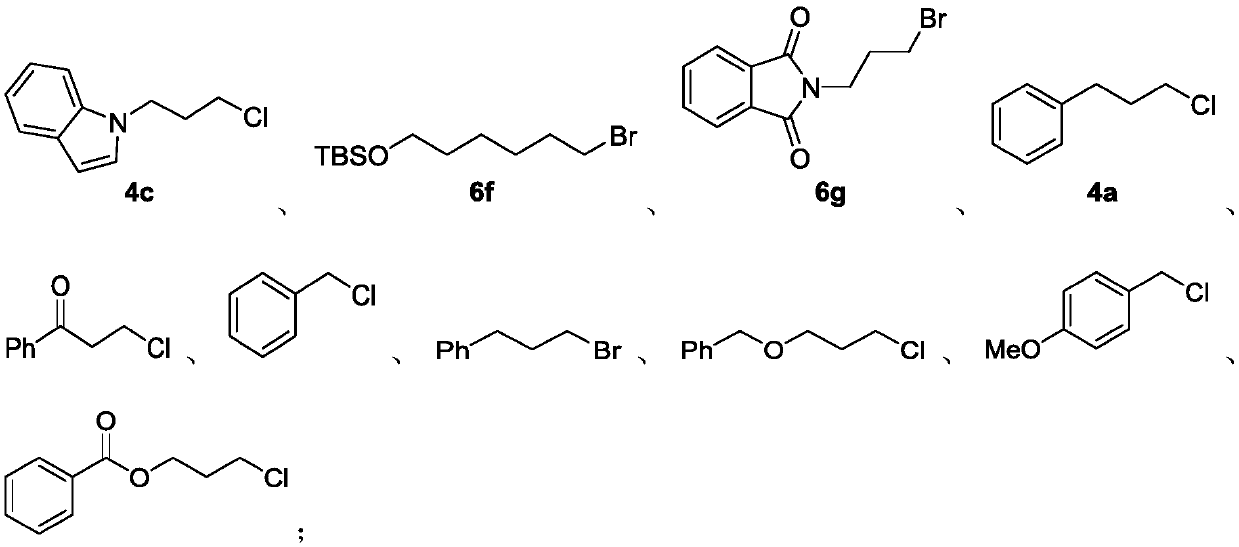
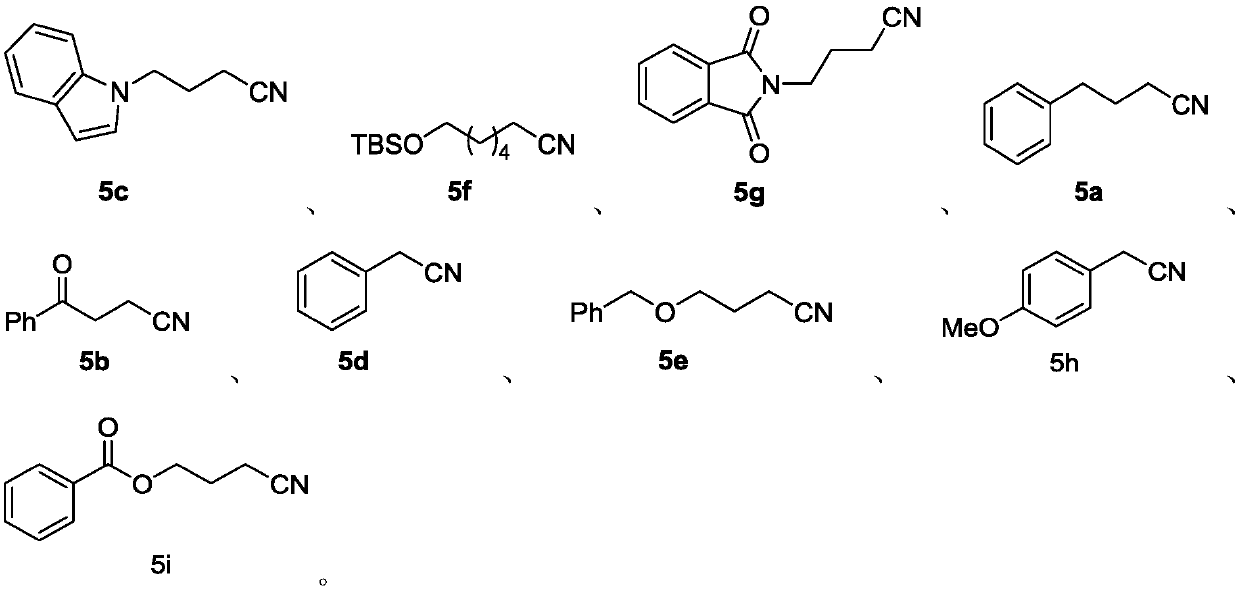
Preparation method of alkyl nitrile compound
A technology of alkyl halide and alkyl nitrile, applied in the field of preparation of alkyl nitrile compounds, can solve the problems of high price, high toxicity of cyanating reagent, unstable catalyst and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 2(6
[0102]
[0103] Under air, add 6-bromo-n-hexanol (1.81g, 10mmol), dichloromethane (20mL), and imidazole (1.36g, 20mmol) successively into the reaction flask equipped with a stirring bar. Slowly added tert-butyldimethylsilyl chloride (2.26g, 15mmol), a large amount of white solid precipitated out, and reacted at room temperature for 7h. The reaction was complete as detected by TLC, the reaction was quenched by saturated ammonium chloride solution, the organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated, silica gel column chromatography, the eluent was petroleum ether:ethyl acetate=100:1, the product It is 2.33g of yellow liquid, the yield is 79%, 1 H NMR purity greater than 98%. 1 H NMR (400MHz, CDCl 3 )δ3.60(t, J=6.4Hz, 2H), 3.40(t, J=6.8Hz, 2H), 1.86(quint, J=6.8Hz, 2H), 1.55-1.31(m, 6H), 0.89( s,9H),0.04(s,6H); 13 C NMR (100MHz, CDCl 3 )δ62.99, 33.88, 32.78, 32.58, 27.95, 25.94, 24.99, 18.33, -5.31.
preparation example 3(6
[0105]
[0106] Under argon, phthalimide (1.47g, 10mmol), potassium carbonate (4.15g, 30mmol), tetrabutylammonium bromide (322.4mg, 1mmol) were sequentially added to the reaction flask equipped with a stirring bar, The gas was exchanged three times under argon, 1,3-dibromopropane (3.0 mL, 30 mmol) was added, and the mixture was reacted at room temperature for 4 h. TLC detects that the reaction is complete, the reaction is quenched with water, the organic phase is extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated, and silica gel column chromatography, the eluent is petroleum ether to petroleum ether: ethyl acetate=5: 1, the product is White solid 1.21g, yield 45%, 1 H NMR purity greater than 98%. 1 H NMR (400MHz, CDCl 3 )δ7.87-7.84(m,2H),7.76-7.73(m,2H),3.86-3.83(m,2H),3.44-3.41(m,2H),2.30-2.23(m,2H); 13 C NMR (100MHz, CDCl 3 )δ168.18, 134.01, 131.90, 123.25, 36.64, 31.56, 29.79.
Embodiment 1
[0107] Example 1 (5a)
[0108]
[0109] In a glove box filled with nitrogen, add Zn(CN) to the 4mL sample bottle in sequence 2 (47.0mg, 0.4mmol), n-Bu 4 NCl (277.9mg, 1.0mmol), cap the bottle, remove from the glove box, add N-methylpyrrolidone (0.5mL) and 4a (77.3mg, 0.5mmol) successively under air, cap the bottle, and place at 140°C Reaction 6h. The reaction was complete as detected by TLC, the reaction was quenched with water, the organic phase was extracted with ether, dried over anhydrous sodium sulfate, concentrated, and subjected to silica gel column chromatography, the eluent was petroleum ether: dichloromethane = 3:1, and the product was 57.3 mg of a colorless liquid , yield 79%, 1 H NMR purity greater than 98%. 1 H NMR (400MHz, CDCl 3 )δ7.32-7.17 (m, 5H), 2.76 (t, J = 7.6Hz, 2H), 2.29 (t, J = 7.2Hz, 2H), 1.99-1.92 (m, 2H); 13 C NMR (100MHz, CDCl 3 )δ139.61, 128.53, 128.33, 126.36, 119.42, 34.22, 26.77, 16.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com