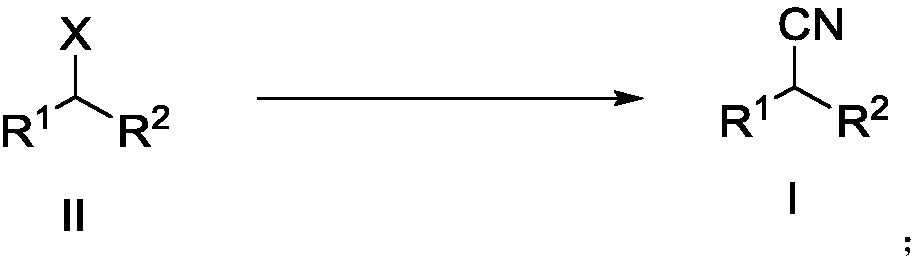
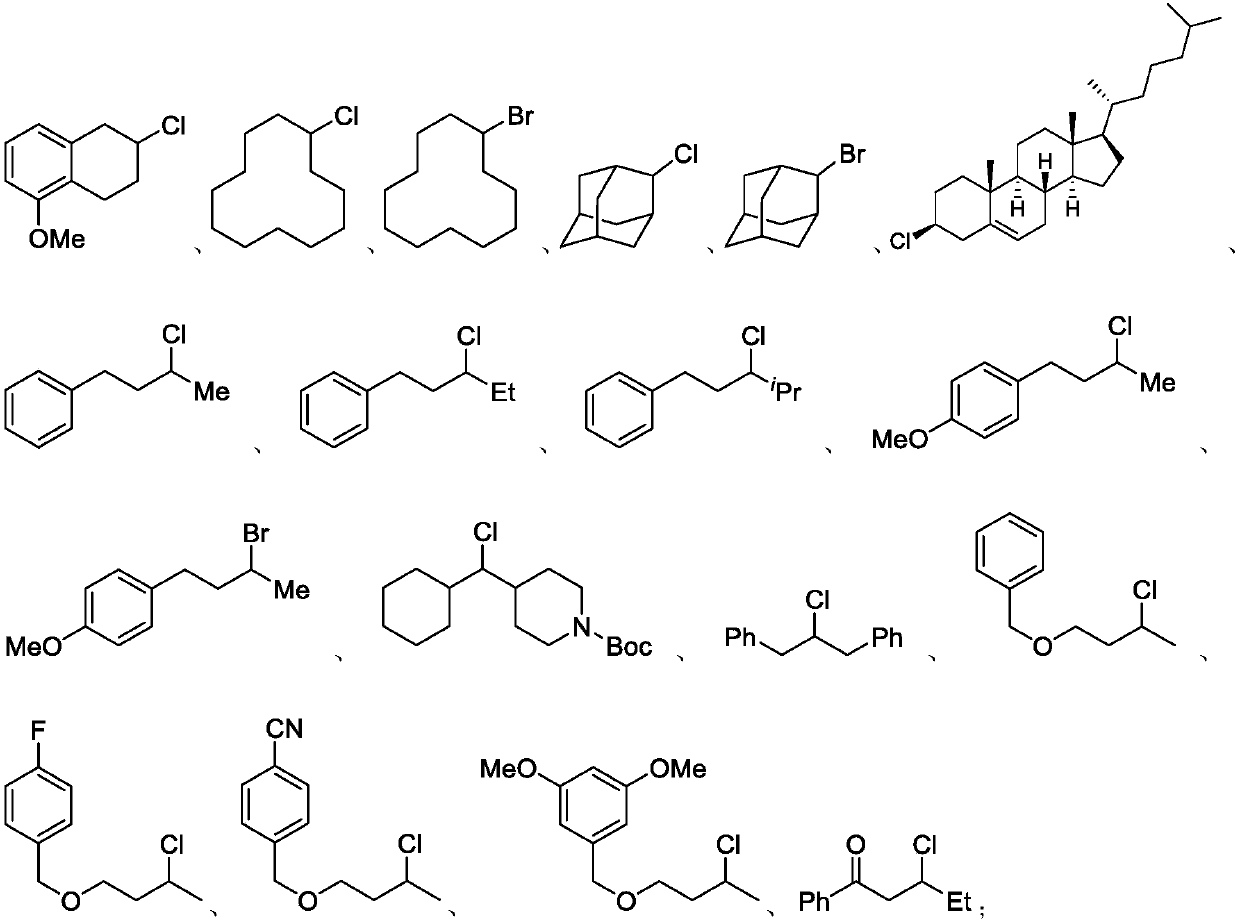
Preparation method of alkyl nitrile compound
A technology of alkyl halides and compounds, which is applied in the field of preparation of alkyl nitrile compounds, and can solve problems such as poor functional group compatibility, high price, and poor substrate universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 6(1
[0132]
[0133] Under the protection of argon, N-benzyl-3-pyrrolidinol (1.06g, 6mmol), toluene (11mL), phosphorus oxychloride (2.3mL, 24.6mmol) were successively added to the reaction flask, heated to reflux for 4h, and detected by TLC The response is complete. The reaction solution was slowly poured into ice water to quench the reaction, the solid potassium carbonate adjusted the pH of the system to alkaline, the organic phase was extracted with ethyl acetate, dried over anhydrous magnesium sulfate, concentrated, silica gel column chromatography, the eluent was n-pentane to ethyl acetate, the product is a yellow liquid 807.4mg, yield 69%, 1 H NMR purity greater than 98%. 1 H NMR (400MHz, CDCl 3 )δ7.31-7.22 (m, 5H), 4.39-4.33 (m, 1H), 3.66 (dd, J = 24.8Hz, 12.8Hz, 2H), 3.07 (dd, J = 10.4Hz, 6.4Hz, 1H) ,2.78-2.61(m,3H),2.45-2.35(m,1H),2.10-2.02(m,1H); 13 C NMR (100MHz, CDCl 3 )δ138.47,128.62,128.24,127.02,63.21,59.93,56.24,52.45,35.80.IR(neat):2794,1495,1453,1377,1350,1...
preparation example 7(1
[0135]
[0136] Under argon protection, add phenylpropanal (1.34g, 10.7mmol) and tetrahydrofuran (20mL) successively in the reaction flask equipped with a stirring bar, ice-water bath, methylmagnesium bromide (2mL, 1M in THF, 12mmol) slowly Add it into the system, return to room temperature and react for 20h, and TLC detects that the reaction is complete. Quench the reaction with saturated ammonium chloride solution, extract the organic phase with dichloromethane, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, concentrate, perform silica gel column chromatography, and the eluent is petroleum ether: ethyl acetate = 10:1 , the product was a colorless liquid, which was directly used in the next reaction.
[0137] Under the protection of argon, the above crude product, triphenylphosphine (5.25g, 20mmol), and dichloromethane (20mL) were added to the reaction flask equipped with a stirrer in sequence, and trichloroacetamide (3.25g , 20mmol), rea...
preparation example 8(1
[0139]
[0140] Silica gel column chromatography, eluent is sherwood oil, product is colorless liquid 851.4mg, two-step total yield 47%, 1 H NMR purity greater than 98%. 1 H NMR (400MHz, CDCl 3 )δ7.30-7.26(m,2H),7.21-7.17(m,3H),3.83-3.77(m,1H),2.91-2.84(m,1H),2.77-2.65(m,1H),2.03- 1.95(m,2H),1.84-1.64(m,2H),1.01(t,J=7.6Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ141.20,128.49,128.42,125.98,64.75,39.72,32.71,31.55,10.85.IR(neat):3060,3027,2967,2939,2874,1603,1496,1454,1381,1301,1237,1030,966,903,836,807,746,698,660. HRMS(EI)C 11 h 15 Cl[M] + : Calculated value: 182.0862, Measured value: 182.0857.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com