Bisamidocyanogen androstanes derivates, preparation method and use thereof
A technology of bisaminoandrostane and derivatives is applied in the directions of steroids, drug combinations, pharmaceutical formulations, etc., to achieve the effects of rapid onset of action, short duration of maintenance, and strong muscle relaxation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
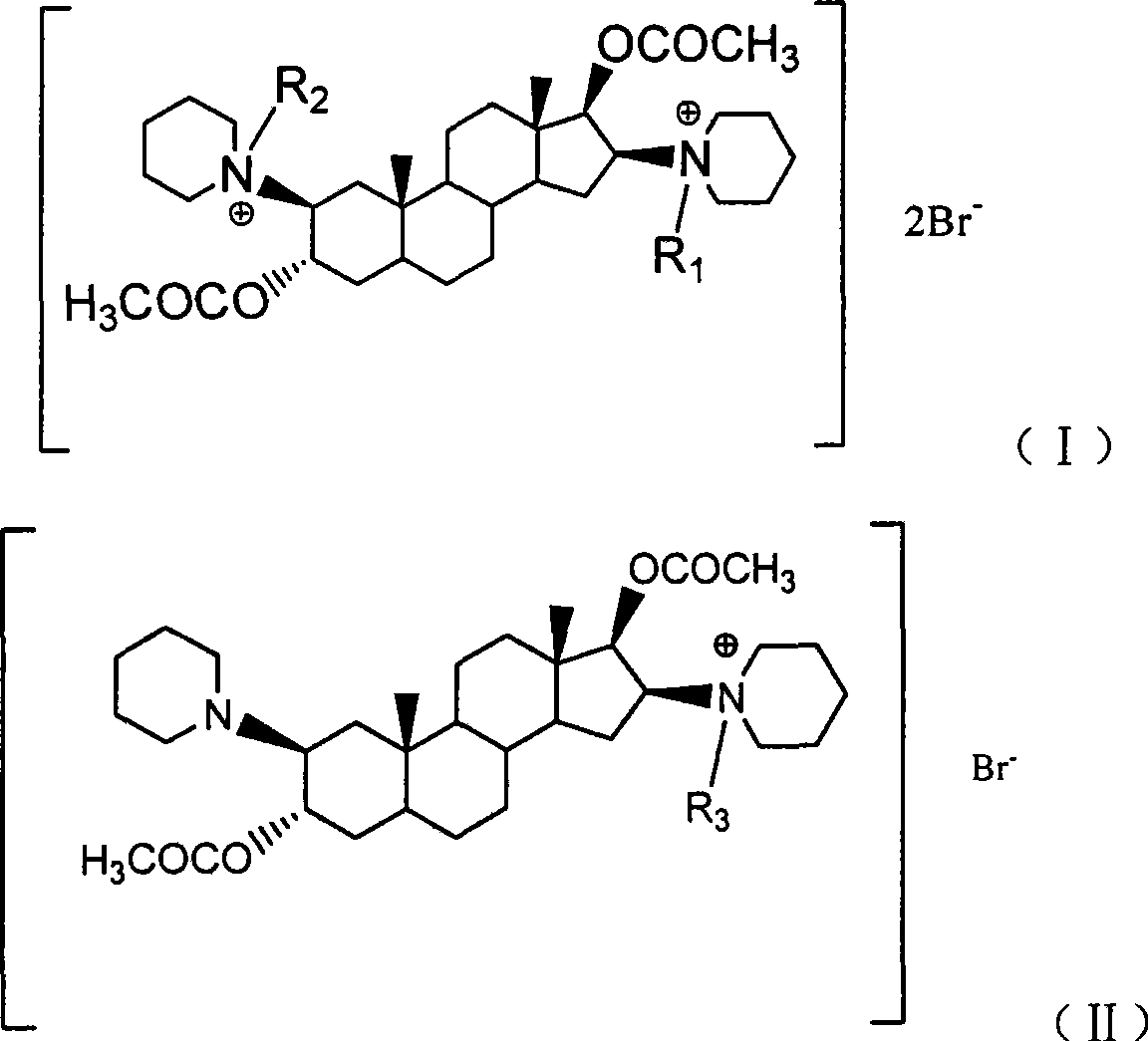
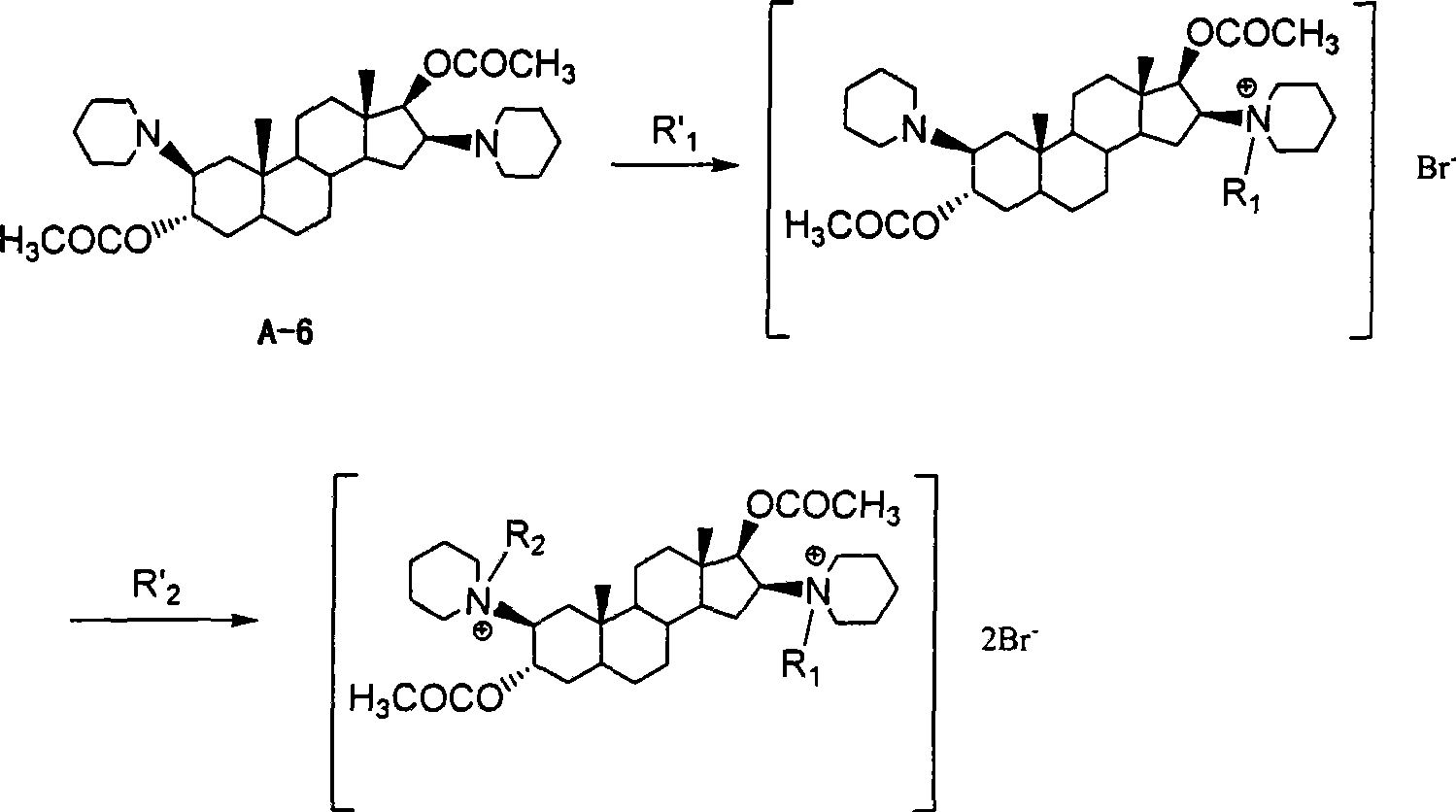
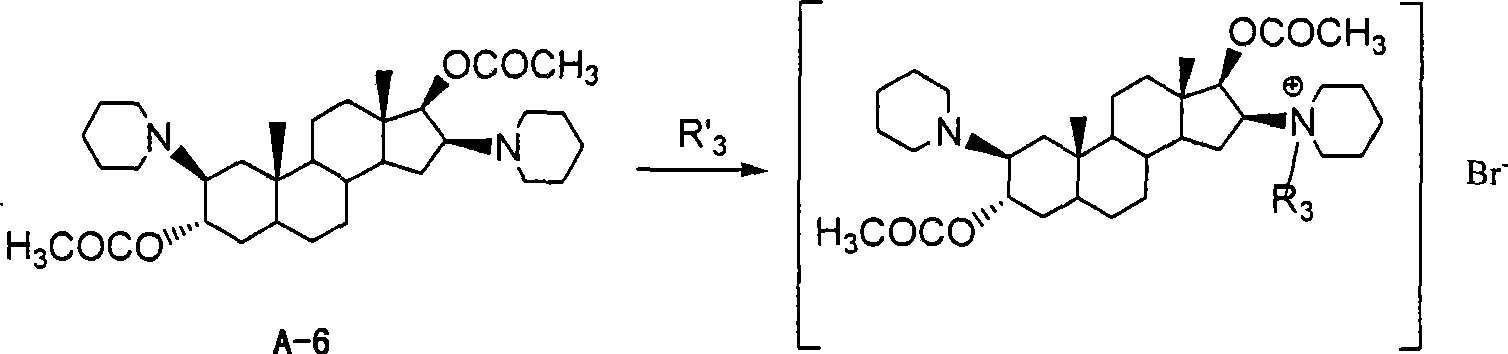
[0024] Example 1: Synthesis of 2β, 16β-bis(1-piperidinyl)-3α, 17β-acetoxy-5α-androstane (A-6)
[0025] Using 5α-androst-2-en-17-one as raw material, it is prepared by esterification of isopropenyl acetate, oxidation of m-chloroperoxybenzoic acid, ring-opening addition with piperidine, reduction of sodium borohydride, and acetylation of acetyl chloride Compound A-6, specific preparation method references: Buckett W.R.; Hewett C.L.; Savage D.S.. Pancuronium Bromide and Other Steroidal Neuromuscular Blocking Agents Containing Acetylcholine Fragments
[0026] [J]. J. Med. Chem, 1973, 16(10): 1116-1124.
Embodiment 2
[0027] Example 2: 1-[3α,17β-diacetoxy-2β(1-piperidinyl)-5α-androstane-16β-yl]-1-acetonitrile piperidine bromide (A-8)
[0028] Dissolve A-6 (4.5g, 8.3mmol) in a mixed solution of acetonitrile (40mL) and acetone (10mL), add bromoacetonitrile (11g, 92mmol), carry out nitrogen protection, stir at room temperature for 40h, and concentrate under reduced pressure. Acidified alumina column chromatography, eluting with isopropanol-ethyl acetate (volume ratio = 2:5) to give an oily product, refined with acetone, and dried to give off-white solid A-8 (3.2 g, yield 51.2%) , mp: 148.8-151.3℃.
[0029]
[0030] 1 HNMR (DMSO-d 6 )H-Hcosy, HSQC δ ppm: 0.70-0.72 (1H, m, 9-position CH, related to 9-position C), 0.75 (3H, s, 18-position CH 3), 0.97 (3H, s, 19 CH 3 ), 0.94-0.99, 1.68-1.71 (2H, m, 28 CH 2 , related to 27, 29 H), 1.21-1.25 (1H, m, 14 CH, related to 8, 15 H), 1.25-1.27 (2H, m, 6 CH 2 , related to 5 and 7 H), 1.24-1.32, 1.69-1.73 (2H, m, 7 CH 2 , related to 6 and 8-positio...
Embodiment 3
[0033] Example 3: 1,1'-[3α,17β-diacetoxy-5α-androstane-2β,16β-diyl]-bis[1-acetonitrile piperidine](A-9) dibromide
[0034] Dissolve A-6 (4.5g, 8.3mmol) in a mixed solution of acetonitrile (40mL) and acetone (10mL), add bromoacetonitrile (21g, 175mmol), under nitrogen protection, stir at about 40°C for 40h, and concentrate under reduced pressure , the product was subjected to alumina (75-240 mesh) column chromatography, eluted with isopropanol-ethyl acetate (volume ratio = 3:1) to obtain an oil, refined with acetone, and dried to obtain an off-white solid A-9 ( 2.8g, yield 43.7%), mp: 147.8-150.9°C.
[0035]
[0036] 1 HNMR (DMSO-d 6 )H-Hcosy, HSQC δ ppm: 0.79 (3H, s, 18 CH 3 ), 0.88-0.99 (1H, m, 9-position CH, related to 9-position C), 0.89 (3H, s, 19-position CH 3 ), 0.88-0.90, 1.63-1.69 (2H, m, 7 CH 2 , related to 6 and 8-bit H), 1.02-1.06 (1H, m, 2-bit-N + CH-), 1.10-1.18, 1.40-1.44 (2H, m, 6 CH 2 , related to 5, 7 H), 1.22-1.33 (1H, m, 14 CH, related to 8, 15 H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com