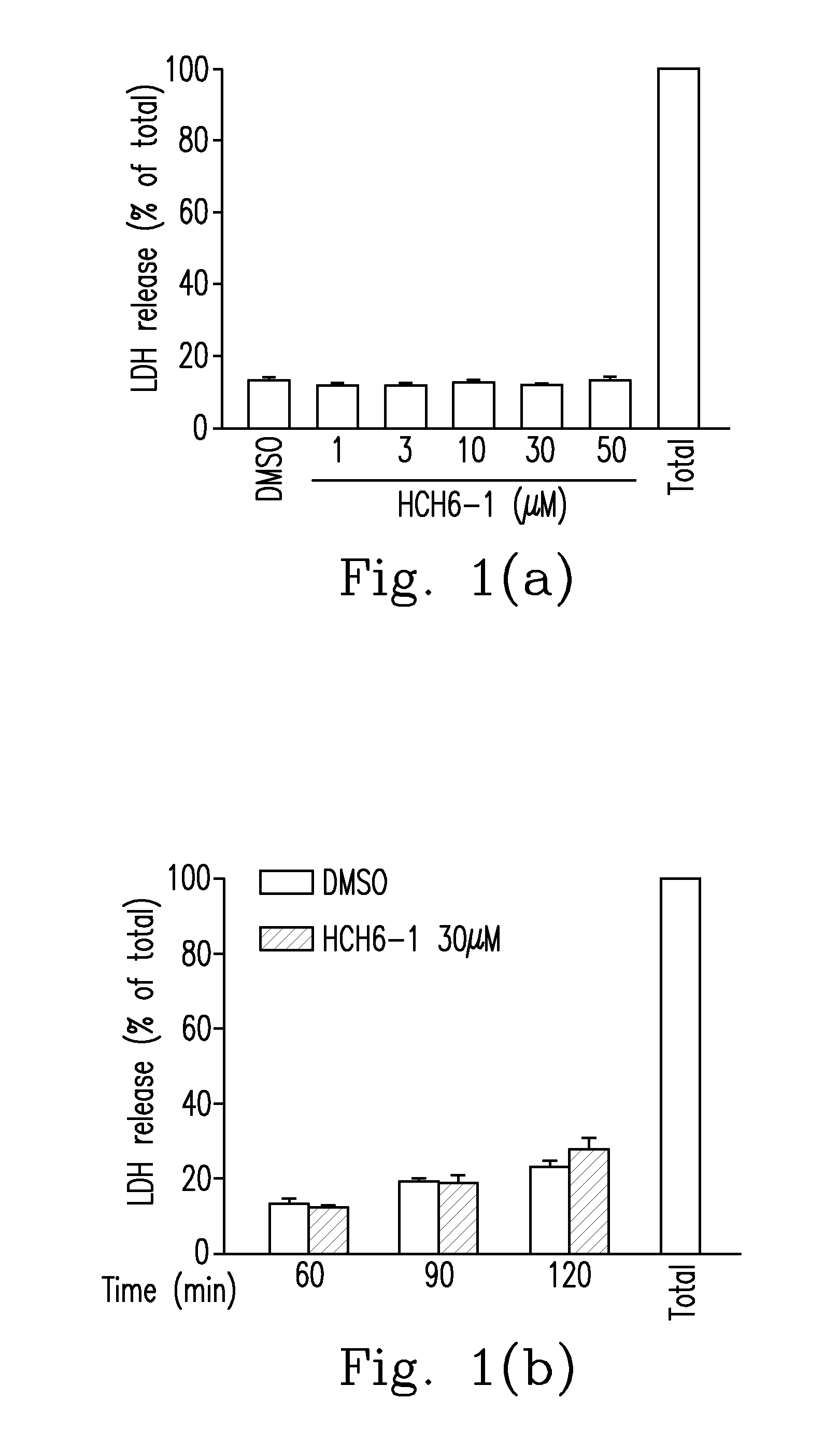
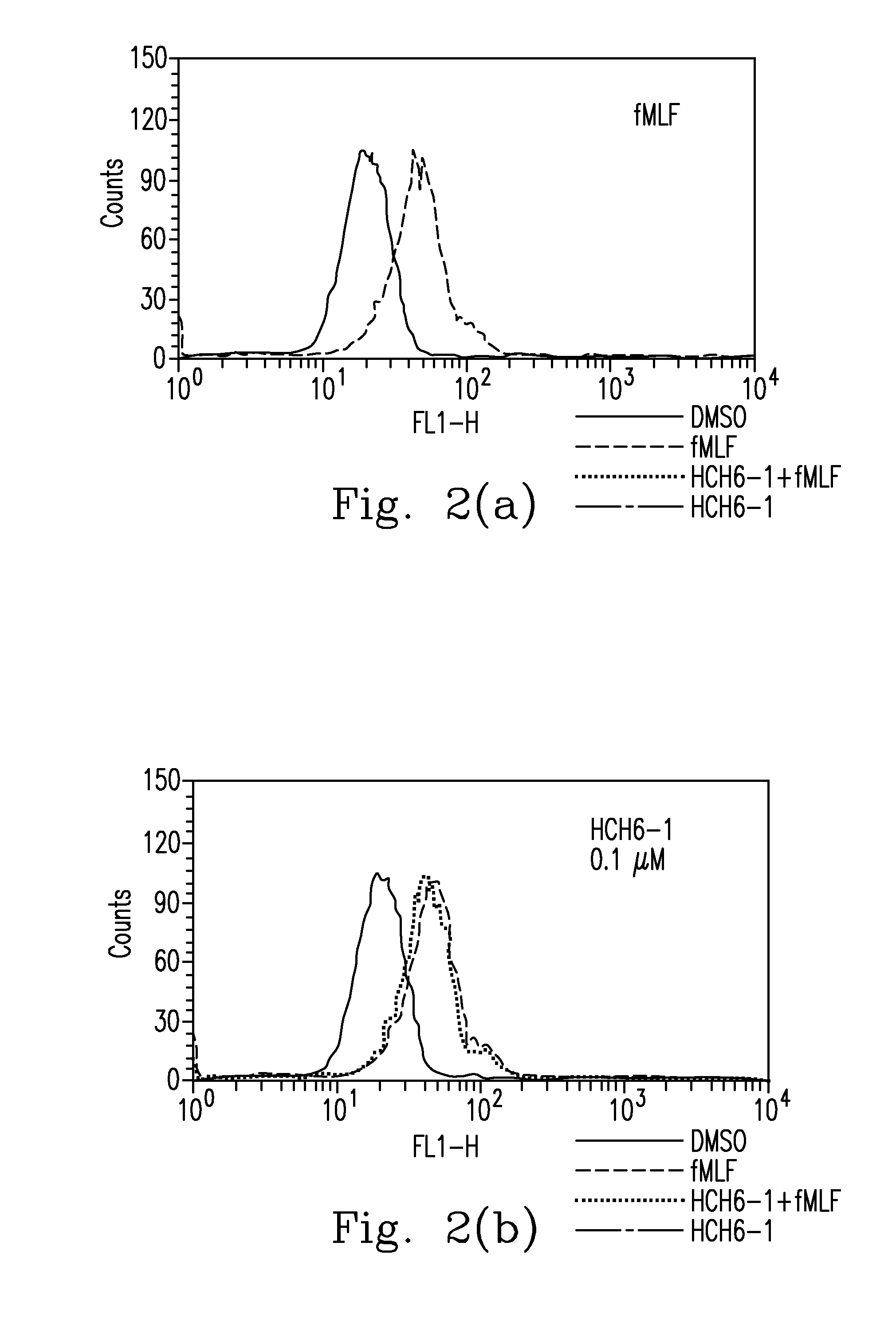
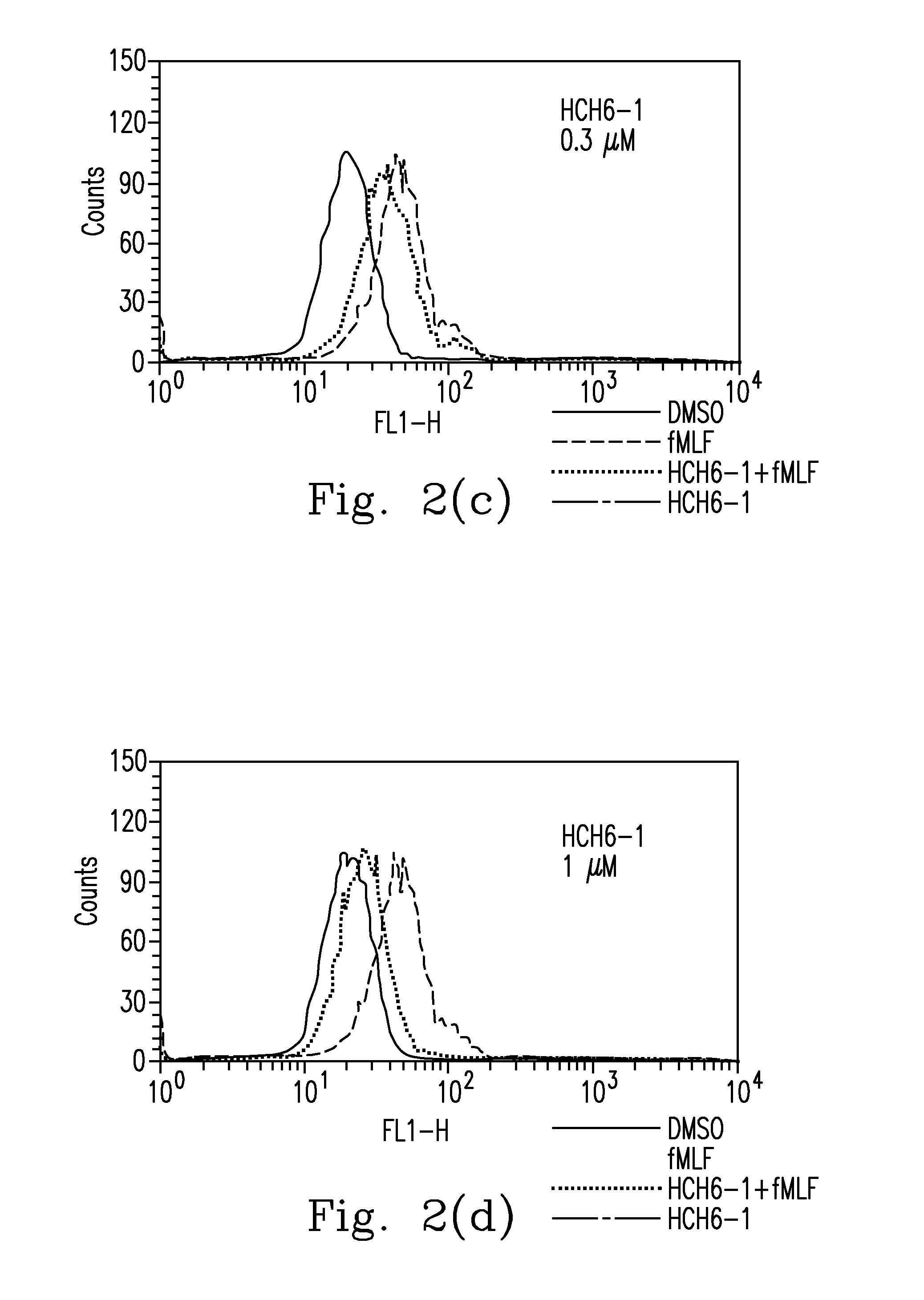
FPR1 antagonist derivatives and use thereof
a technology of fpr1 and derivatives, applied in the field of dipeptide derivatives, can solve problems such as inability to inhibi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
embodiment 1
General Procedure for the Synthesis of N—(N-aroyl-L-tryptophanyl)-D-phenylalanine methyl esters (compounds 3-7) and their analogs (compounds 8-10, 11a and 11 b)
[0074]To a mixture solution of L-tryptophan (2a, 1.0 equiv.) or 5-hydroxy-L-tryptophan (2b, 1.0 equiv.) in 2 N NaOH aqueous solution was added suitable acyl chlorides (1.1 equiv.), respectively. The reaction mixture was stirred at room temperature for 3.0 h, and then 1 N HCl solution was added and the pH values were adjusted to 1-2. The mixture solution was further partitioned by chloroform, and the organic layer was evaporated at reduced pressure to yield intermediates. The intermediates and D-phenylalanine methyl ester (1.0 mmole) were dissolved in DCM, and then HBTU (2.0 equiv.) and DIEA (1.5 equiv.) were added. The reaction mixture was stirred for 6 hours at room temperature, concentrated, and purified by silica gel column chromatography using a mixture of n-hexane-ethyl acetate (6:4) or n-hexane-acetone (7:3), respective...
embodiment 2
General procedure for the synthesis of N—(N-benzoyl-L-tryptophanyl)-D-phenylalaninol derivatives (compounds 12a-13a and 12b-13b)
[0085]N-Benzoyl-L-tryptophan and N-benzoyl-5-hydroxy-L-tryptophan were obtained by a similar procedure as described above. Dissolve N-benzoyl-L-tryptophan (1.0 equiv.) or N-benzoyl-5-hydroxy-L-tryptophan (1.0 equiv.) in DCM respectively, and then D-phenylalaninol (1.0 equiv.), HBTU (2.0 equiv.) and DIEA (1.5 equiv.) were added. The reaction mixture was stirred at room temperature for 6.0 h, and purified by silica gel column chromatography using ethyl acetate or MeOH—CHC13 (1:20) to afford the mixtures 12a / b and 13a / b. The mixture was further purified by HPLC (mobile phase: 35% acetonitrile+0.3% TFA) to afford products.
N—(N-Benzoyl-L-tryptophanyl)-5-hydroxy-D-phenylalaninol (compounds 12a and 12b)
Compound 12a
[0086]16% yield. White powder, mp 175-177° C. 1H NMR (C5D5N) δ 11.77 (1H, s, NH), 8.97 (1H, d, J=8.0 Hz, NH), 8.88 (1H, d, J=8.0 Hz, NH), 8.05 (2H, d, J...
embodiment 3
Preparation of N—(N-nicotinoyl-L-tryptophanyl)-D-phenylalanine methyl esters (compounds 15a and 15b)
[0090]A mixture solution of L-tryptophan methyl ester (1.0 equiv.) in pyridine and nicotinoyl chlorides (1.1 equiv.) was prepared. The reaction mixture was stirred at room temperature for 16.0 h, and then evaporated and purified by silica gel column chromatography using a mixture of MeOH—CHCl3 (1:20) to afford N-nicotinoyl-L-tryptophan methyl ester. N-Nicotinoyl-L-tryptophan methyl ester was further dissolved in 10 mL of 1.0 M LiOH solution, and then added to the mixture for hydrolysis. Upon completion, the reaction mixture was partitioned for three times with ethyl acetate and saturated sodium bicarbonate aqueous solution. The combined aqueous layer was neutralized with 1.0 N HCl solution, followed by extraction with ethyl acetate for three times. The combined organic layer was dried with anhydrous magnesium sulfate and evaporated to yield N-nicotinoyl-L-tryptophan. D-Phenylalanine m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com