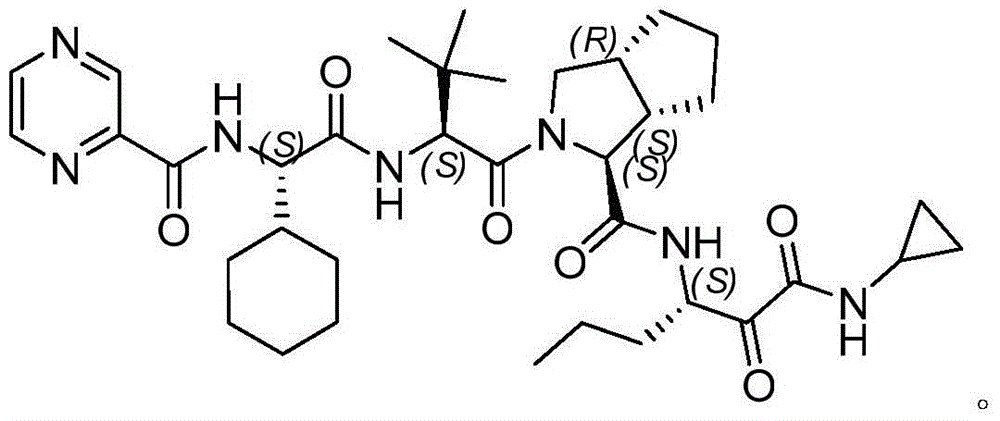
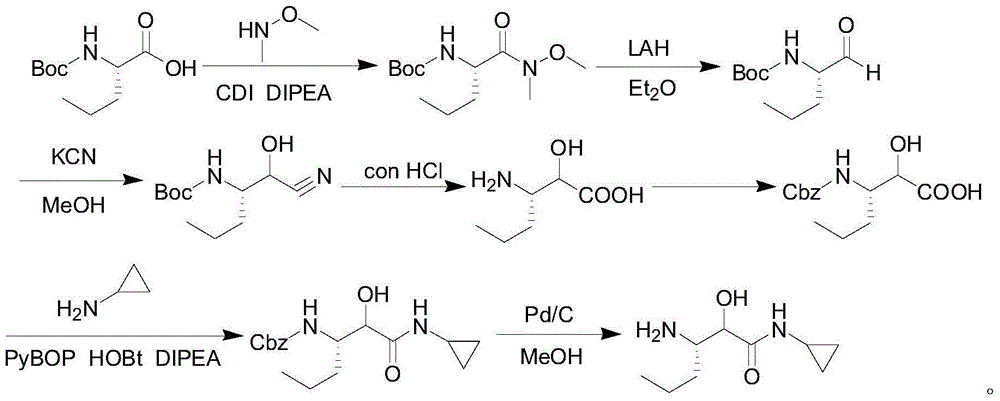
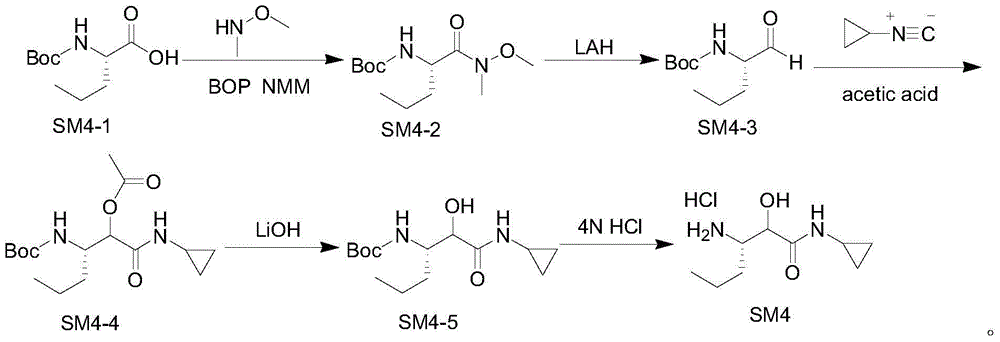
Intermediate for preparing N-cyclopropyl-(2S, 3S)-3-amino-2-hydroxy caproamide, preparation method and applications thereof
A technology of hydroxycaproylamide and cyclopropyl, which is applied in the field of medicinal chemistry, can solve problems such as non-industrialized production routes, failure to meet safety and environmental protection requirements, and unstable product quality control, and achieve simple preparation process, high yield, and low raw material price. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: preparation formula I compound
[0041]
[0042] Dissolve the compound of formula II (20.0 g, 0.15 mol) in 100 mL of acetonitrile, and slowly add boron trifluoride ether (28.0 g, 0.20 mol) dropwise under ice bath. to room temperature, stirred until the compound of formula II reacted completely (about 6 hours); under ice bath, slowly added saturated aqueous sodium bicarbonate solution to adjust the pH value to 5-6; concentrated under reduced pressure at 45-50°C, and concentrated The residue was extracted three times with dichloromethane (120 mL×3), the organic phase was collected, concentrated under reduced pressure, and the concentrated residue was subjected to column chromatography to obtain 20.3 g of the compound of formula I with a molar yield of 77.2%.
[0043] 1 H NMR (DMSO-d 6 300MHz)δ11.01(br s,1H),4.40(d,J=6.3Hz,1H),4.12(dd,J=6.9,6.3Hz,1H),1.33-1.41(m,4H),1.16(s ,3H),0.91(t,J=7.2Hz,3H);
[0044] MS(ESI): m / z=172.1[M+H] + , 170.1 [M-H].
Embodiment 2
[0045] Embodiment 2: preparation formula III compound
[0046]
[0047] Add 20.3g of the compound of formula I into 80mL of a mixed solvent formed by concentrated hydrochloric acid and water at a volume ratio of V / V=1 / 1, heat to reflux, and reflux until the compound of formula I reacts completely (about 4 hours); concentrate the reaction solution , stripped twice with ethanol (40mL×2) to obtain the mixed compound of formula III (light yellow liquid, the ratio of (S,S)-configuration to (R,R)-configuration determined by HPLC is 89:11) 15.7g, the molar yield is 90.1%.
[0048] MS(ESI): m / z=148.1[M+H] + , 146.1[M-H] - .
Embodiment 3
[0049] Embodiment 3: preparation formula IV compound
[0050]
[0051]Sodium carbonate (6.9g, 0.065mol) and the mixed compound of formula III (8.0g, 0.054mol) were dissolved in 80mL of a mixed solvent formed by acetonitrile and water by volume ratio V / V=1 / 1, stirred at room temperature , add dicarbonyl di-tert-butyl ester (14.2g, 0.065mol) in batches, after the addition is complete, stir the reaction at room temperature until the reaction of the compound of formula III is complete (about 6 hours); filter with suction, and wash the filter cake with acetonitrile for two (8mL×2), the filtrate was concentrated under reduced pressure; under ice bath, slowly add glacial acetic acid dropwise to the concentrate to adjust the pH value to 5-6; After filtration, the filter cake was washed twice with water (8 mL×2), and dried to obtain 12.1 g of the compound of formula IV as a white solid, with a molar yield of 90.0%.
[0052] MS(ESI): m / z=248.1[M+H] + , 246.1[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com