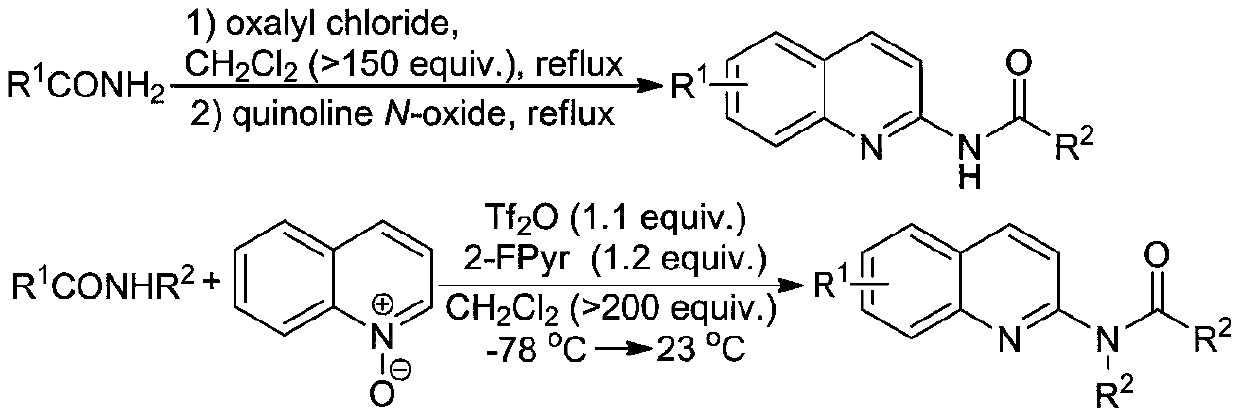
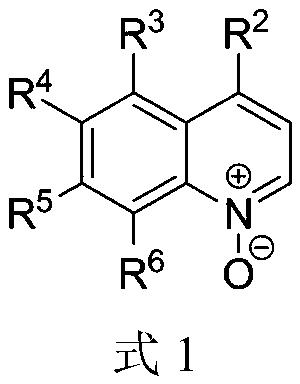
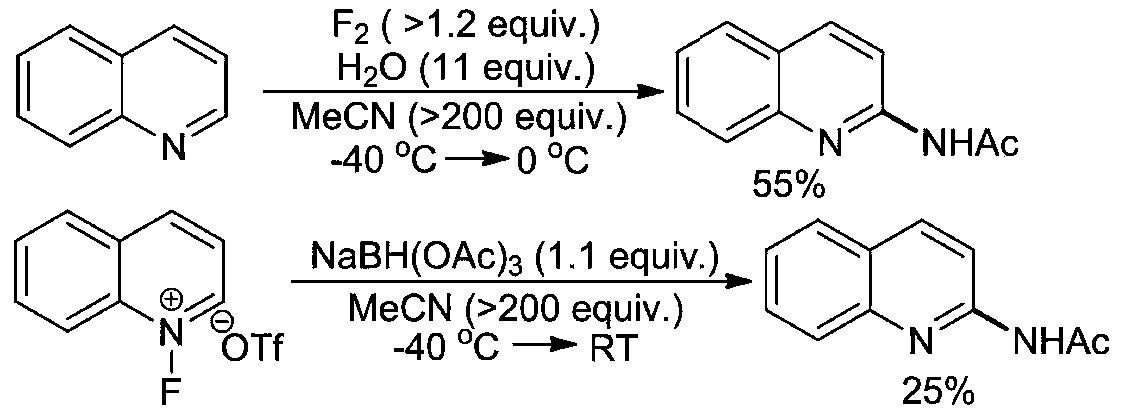A kind of preparation method of ultrasonic-assisted n-(quinolin-2-yl) alkyl amides
An ultrasonic-assisted, alkyl amide technology, applied in organic chemistry, etc., can solve problems such as increased reaction costs, environmental side effects, harsh reaction conditions, etc., and achieve the effects of improved synthesis efficiency, simple reaction conditions, and easy-to-source sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of N-(quinolin-2-yl)acetamide:
[0067] In the 15mL round bottom flask, add quinoline nitrogen oxide 1.45g (10mmol) successively, acetonitrile 2.2mL (2.16g, 50mmol), the 732 cation exchange resins of 5% mass fraction, the ultrasonic reaction device of gained mixture is in 30W / 40KHz Medium ultrasonic reaction for 30 minutes. The resin catalyst in the reaction system was recovered by filtration, the unreacted acetonitrile in the reaction system was recovered under reduced pressure, and 1.72 g of the corresponding acetamide was obtained by recrystallization, with a yield of 93%.
Embodiment 2
[0075] Use 15W ultrasonic power instead of 30W ultrasonic power to assist the reaction:
[0076] In a 15mL round-bottomed flask, add quinoline nitrogen oxide 1.45g (10mmol) successively, acetonitrile 2.2mL (2.16g, 50mmol), the 732 cation exchange resins of 5% mass fraction, the ultrasonic reaction device of gained mixture at 15W / 40KHz Medium ultrasonic reaction for 60 minutes. Thin-layer chromatographic analysis confirmed that part of the quinoline raw material had not reacted completely. The resin catalyst in the reaction system was recovered by filtration, and the yield of N-(quinolin-2-yl)acetamide was calculated to be 68% by the liquid chromatography area normalization method.
Embodiment 3
[0078] Use 60W ultrasonic power instead of 30W ultrasonic power to assist the reaction:
[0079] In the 15mL round bottom flask, add quinoline nitrogen oxide 1.45g (10mmol) successively, acetonitrile 2.2mL (2.16g, 50mmol), the 732 cation exchange resins of 5% mass fraction, the ultrasonic reaction device of gained mixture is in 60W / 40KHz Medium ultrasonic reaction for 30 minutes. The resin catalyst in the reaction system was recovered by filtration, the unreacted acetonitrile in the reaction system was recovered under reduced pressure, and 1.72 g of the corresponding acetamide was obtained by recrystallization, with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com