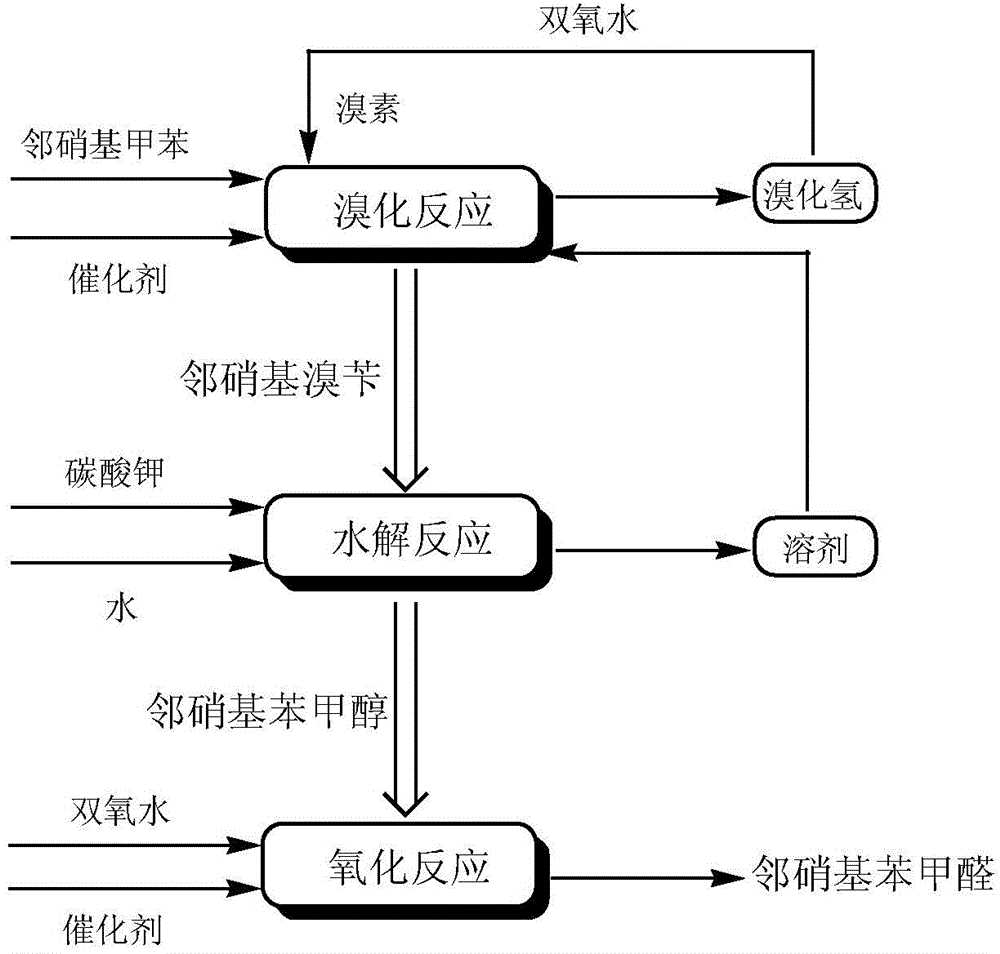
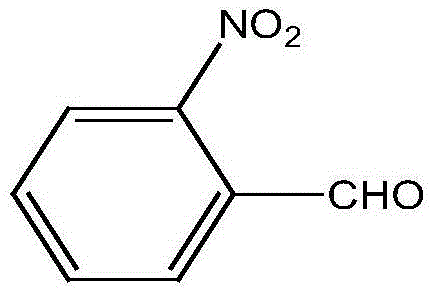
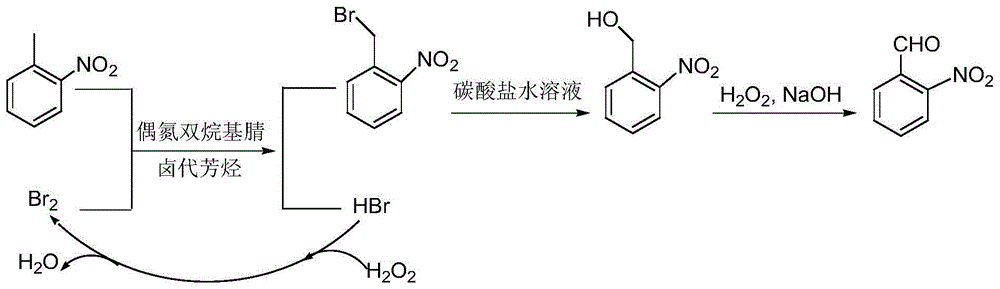
A preparing method of 2-nitrobenzaldehyde
A technology of o-nitrobenzaldehyde and azobisalkyl nitrile, applied in the field of preparation of known compounds, can solve problems such as production cost and industrial operation not having obvious advantages, difficult to regenerate metal oxides, poor thermal stability and the like , to reduce the generation of organic by-products, reduce the cost of industrial preparation, and avoid pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the reactor, add 100g of chlorobenzene, 16.4g of o-nitrotoluene, 3.3g of azobisisovaleronitrile, slowly add 11.5g of bromine dropwise within 30min at 50°C, after the dropwise addition, stir and heat to reflux React until the bromine color fades. Slowly add 8.5 g of 27.5% hydrogen peroxide dropwise within 30 min, and stir and reflux for 2 h. Add 54.7g of 30% potassium carbonate aqueous solution to the reaction solution, stir and reflux for 16 hours, recover chlorobenzene by distillation, cool and stand to separate the aqueous phase and the organic phase containing o-nitrobenzyl alcohol. Transfer the organic phase into the reactor, add 3.2g of sodium hydroxide, stir and control the temperature at 30°C, add 58.5g of 35% hydrogen peroxide dropwise within 30min, and heat to reflux for 23h. After the reaction was completed, it was cooled to room temperature, and the organic phase was separated by standing. After washing, the organic solvent was concentrated, and 13.6 g of...
Embodiment 2
[0031] In the reactor, add 100g of chlorobenzene, 16.4g of o-nitrotoluene, and 1.5g of azobisisoheptanonitrile, and slowly add 10.8g of bromine dropwise within 30 minutes at 45°C. After the dropwise addition, stir and heat to reflux to react until the bromine color fades. Slowly add 8.5 g of 27.5% hydrogen peroxide dropwise within 30 min, and stir and reflux for 2 h. Add 60.0 g of 30% potassium carbonate aqueous solution to the reaction solution, stir and reflux for 16 hours, recover chlorobenzene by distillation, and cool and stand to separate the aqueous phase and the organic phase containing o-nitrobenzyl alcohol. Transfer the organic phase into the reactor, add 1.5g of sodium hydroxide, stir and control the temperature at 30°C, add 75.0g of 27.5% hydrogen peroxide dropwise within 30min, and heat to reflux for 23h. After the reaction was completed, cool to room temperature, stand to separate the organic phase, concentrate the organic solvent after washing, and refine with ...
Embodiment 3
[0033]In the reactor, add 100g of o-dichlorobenzene, 16.4g of o-nitrotoluene, 1.5g of azobisisooctanonitrile, and slowly add 10.8g of bromine dropwise within 30min at 45°C. After the dropwise addition, stir and heat The reaction was refluxed until the color of bromine faded. Slowly add 8.5 g of 27.5% hydrogen peroxide dropwise within 30 min, and stir and reflux for 2 h. Add 60.0 g of 30% potassium carbonate aqueous solution to the reaction solution, stir and reflux for 16 hours, recover ortho-dichlorobenzene by distillation, and cool and stand to separate the water phase and the organic phase containing o-nitrobenzyl alcohol. Transfer the organic phase into the reactor, add 1.5g of sodium hydroxide, stir and control the temperature at 30°C, add 41.3g of 50% hydrogen peroxide dropwise within 30min, and heat to reflux for 23h. After the reaction was completed, it was cooled to room temperature, and the organic phase was separated by standing. After washing, the organic solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com