Preparation method of 2-phenyl substituted pyridine nitrogen oxide compound
A technology for pyridine nitrogen oxide compounds and pyridine nitrogen oxide derivatives, which is applied in the field of preparation of pyridine nitrogen oxide derivatives, can solve the problems of harsh reaction conditions, poor regioselectivity, flammable catalysts and the like, and achieves simple operation, potential biological activity, The effect of catalyst safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
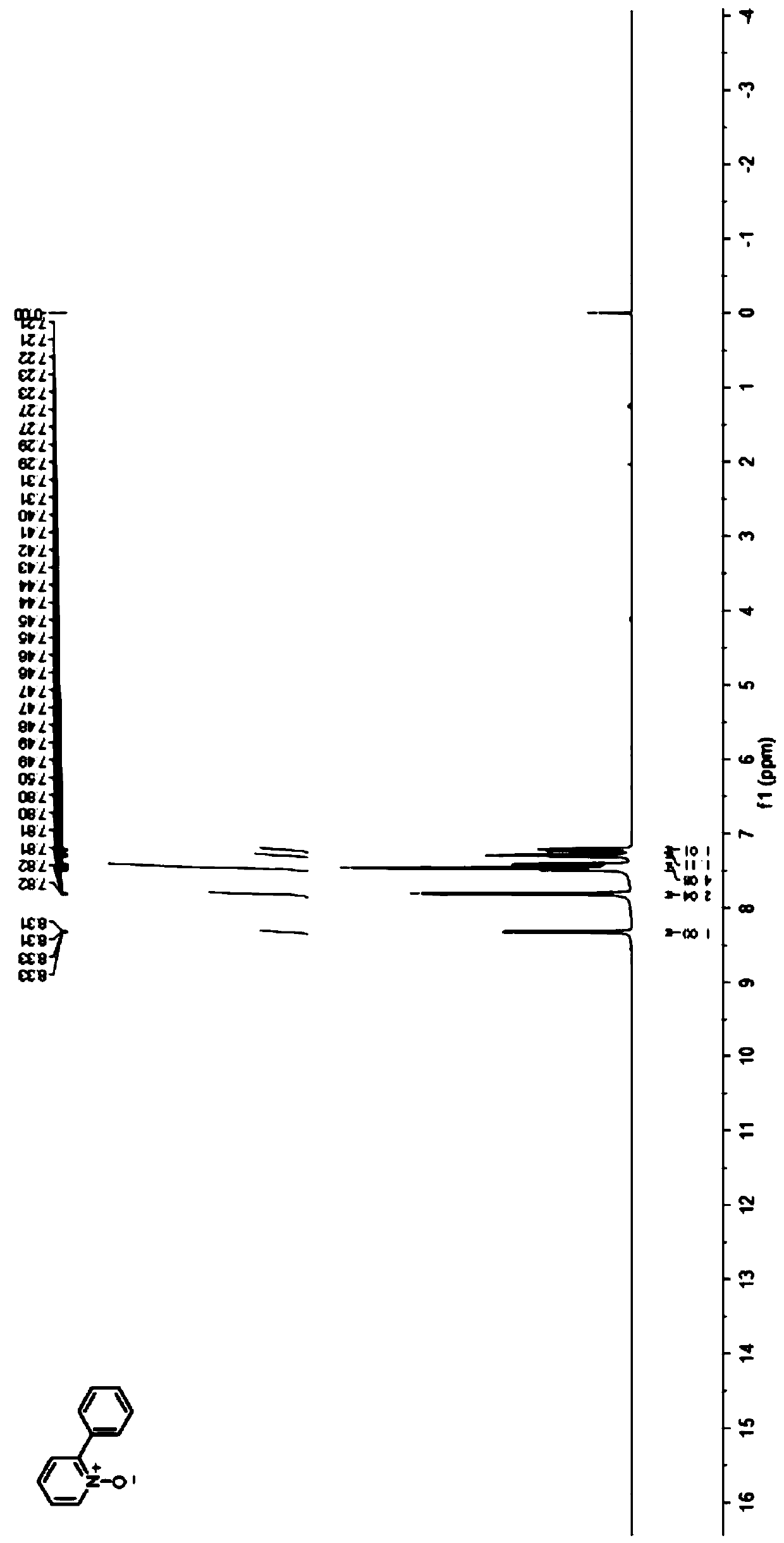
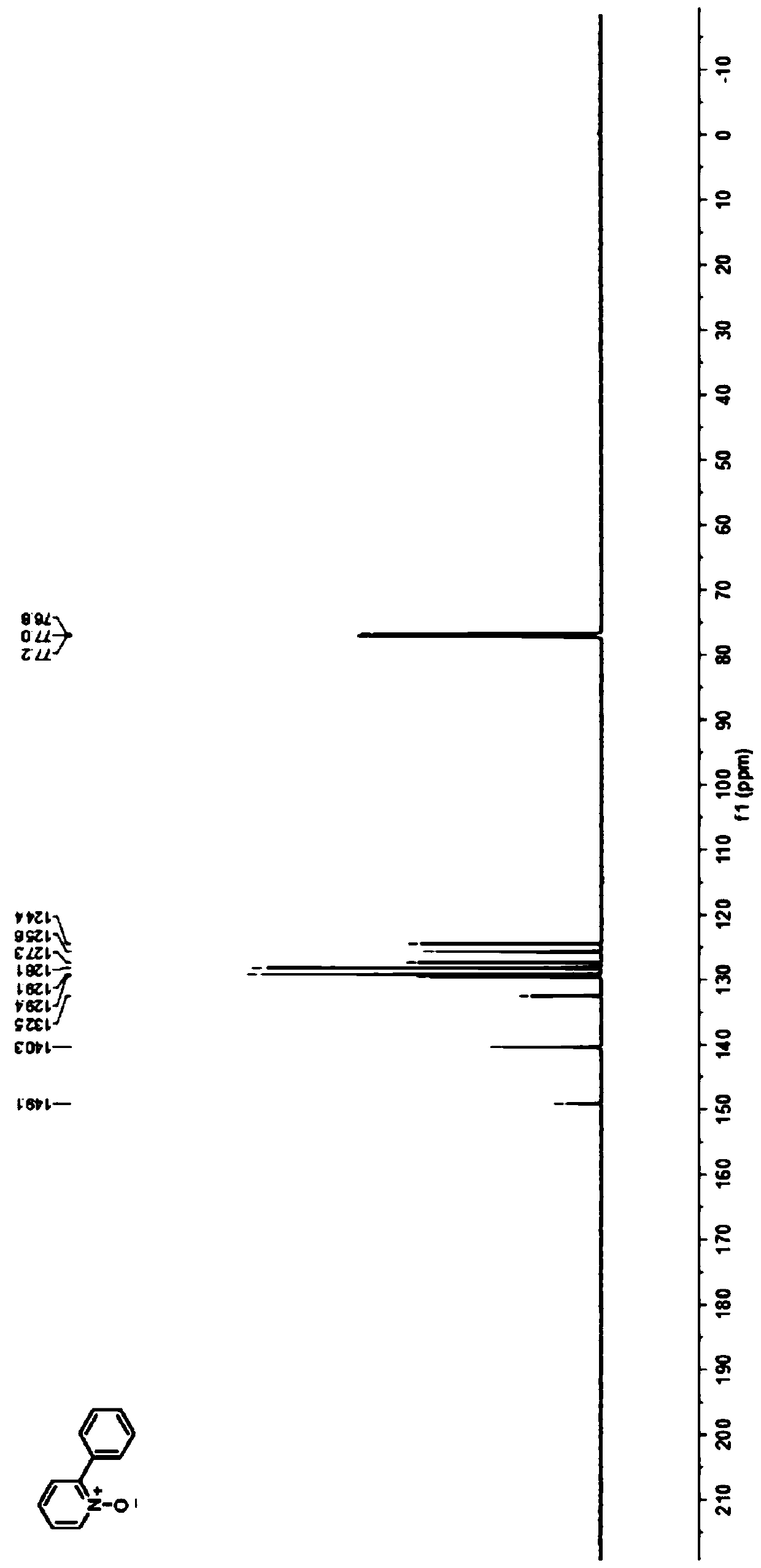
[0029] Embodiment 1: The preparation method of the 2-phenyl substituted pyridine nitrogen oxide compound of the present embodiment 1 is carried out according to the following steps:
[0030] 1. At room temperature, mix 9.5mg (0.1mmol) pyridine nitrogen oxide, 73.6mg (0.2mmol) diphenyl iodine tetrafluoroborate, 7.1mg (10mol%) eosin Y photocatalyst, 32.5mg (0.1mmol) Cesium carbonate and 54.1 mg (0.2 mmol) K 2 S 2 o 8 Add it into a 10mL penicillin bottle, seal it with a rubber stopper and a parafilm; then use a needle to connect the penicillin bottle to an air pump, pump out the air in the penicillin bottle, backfill it with nitrogen, repeat three times, a nitrogen atmosphere is formed in the penicillin bottle, and then inject 1mL of methanol As a solvent, mix well; at room temperature, the reactor is illuminated with 5W blue LEDs for reaction, and the reaction progress is monitored by TLC, and the reaction is completed after 3 days;
[0031] Two, after the reaction finishes, ...
Embodiment 2
[0039] Embodiment 2: The preparation method of the 2-phenyl substituted pyridine nitrogen oxide compound of this embodiment is carried out according to the following steps:
[0040] 1. At room temperature, mix 14.0mg (0.1mmol) 4-nitropyridine nitroxide, 73.6mg (0.2mmol) diphenyl iodine tetrafluoroborate, 7.1mg (10mol%) eosin Y photocatalyst, 32.5mg (0.1mmol) cesium carbonate and 54.1mg (0.2mmol) K 2 S 2 o 8 Add it into a 10mL penicillin bottle, seal it with a rubber stopper and a parafilm; then use a needle to connect the penicillin bottle to an air pump, pump out the air in the penicillin bottle, backfill it with nitrogen, repeat three times, a nitrogen atmosphere is formed in the penicillin bottle, and then inject 1mL of methanol As a solvent, mix well; at room temperature, the reactor is illuminated with 10W blue LEDs light for reaction, and the reaction progress is monitored by TLC, and the reaction is completed after 2 days of reaction;
[0041]Two, after the reaction ...
Embodiment 3
[0050] Embodiment 3: The preparation method of the 2-phenyl substituted pyridine nitrogen oxide compound of this embodiment is carried out according to the following steps:
[0051] 1. At room temperature, 12.0mg (0.1mmol) of 4-cyanopyridine nitroxide, 73.6mg (0.2mmol) of diphenyl iodine tetrafluoroborate, 7.1mg (10mol%) of eosin Y photocatalyst, 32.5mg (0.1mmol) cesium carbonate and 54.1mg (0.2mmol) K 2 S 2 o 8 Add it into a 10mL penicillin bottle, seal it with a rubber stopper and a parafilm; then use a needle to connect the penicillin bottle to an air pump, pump out the air in the penicillin bottle, backfill it with nitrogen, repeat three times, a nitrogen atmosphere is formed in the penicillin bottle, and then inject 1mL of methanol As a solvent, mix well; at room temperature, the reactor is illuminated with 15W blue LEDs light for reaction, and the reaction progress is monitored by TLC, and the reaction is completed after 2 days of reaction;
[0052] Two, after the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com