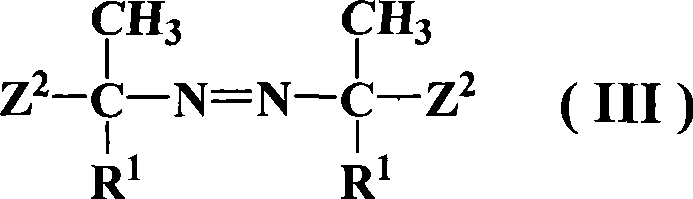
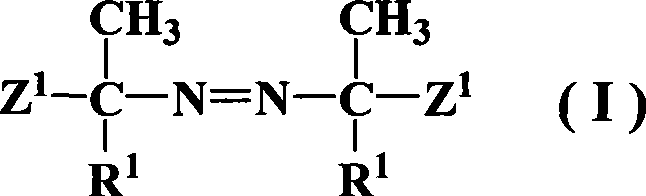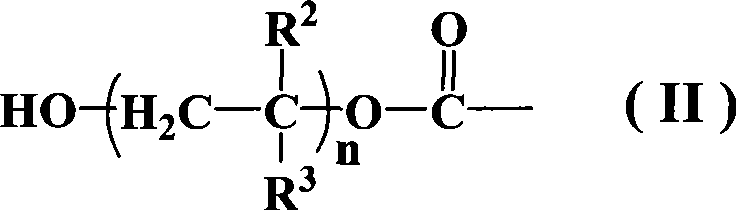Azos thermal initiator, synthetic method and application thereof
A technology of thermal initiator and synthesis method, applied in organic chemistry and other directions, can solve problems such as expensive and difficult to realize industrialized production, and achieve the effects of controllable initiation rate, high initiation efficiency and high grafting rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of intermediates of azo derivatives with hydroxyl groups at the end
[0037] Put 150ml of benzene, 150ml of ethylene glycol, and 22g of azobisisobutyronitrile into a 500ml flask. Under the condition of mechanical stirring, dry hydrogen chloride gas was introduced slowly and continuously, and reacted at 5°C for 10 hours. At the end of the reaction, the reactant turned into a white viscous liquid. Add 15g of ice cubes to the white viscous liquid and stir vigorously for 2 hours to fully hydrolyze it.
[0038] Use a separatory funnel to separate the viscous material in the lower layer, collect the upper benzene layer; then wash with 1200ml saturated sodium chloride solution for 3 times, then wash with 900ml saturated sodium bicarbonate solution for 3 times, add anhydrous magnesium sulfate to dry; The solvent benzene was evaporated under pressure, and then 20ml of methanol was added to precipitate a small amount of white oligomer. After the methanol was ...
Embodiment 2
[0039] Embodiment 2 prepares thermal initiator by intermediate
[0040] 4.0 ml of methacryloyl chloride, 6.0 g of the intermediate and 50 ml of benzene were added to a vessel equipped with a magnetic stirrer. Then, 3.6ml of pyridine was slowly added dropwise, and the dropping process was cooled with an ice-water bath. After the dropwise addition, the container was sealed and reacted at room temperature for three days.
[0041] The pyridinium salt was removed by filtration, washed three times with 900 ml of saturated sodium chloride solution, and dried over anhydrous magnesium sulfate. The crude product was separated and purified by silica gel column chromatography, and the eluent was a mixed solvent of benzene:acetone=2:1 (volume ratio). Finally, 0.9 g of product was obtained with a purity of 96%.
Embodiment 3
[0042] Example 3 Preparation of Thermal Initiator by Intermediate
[0043] In a container equipped with magnetic stirring, 4.8ml of pyridine was slowly dropped into 5.6ml of methacryloyl chloride, and the dropping process was cooled with an ice-water bath. Then add 43ml of acetone and accelerate the stirring to disperse the generated pyridinium salt evenly. Then slowly add 8.7g of the intermediate dropwise, and cool with an ice-water bath during the dropwise addition. After the dropwise addition, the container was sealed and reacted at room temperature for three days.
[0044] The pyridinium salt was removed by filtration, the acetone was evaporated under reduced pressure, and 50 ml of benzene was added as a solvent. Wash with 900 ml of saturated sodium chloride solution three times, and dry over anhydrous magnesium sulfate. The crude product was separated and purified by silica gel column chromatography, and the eluent was a mixed solvent of benzene:acetone=2:1 (volume rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com