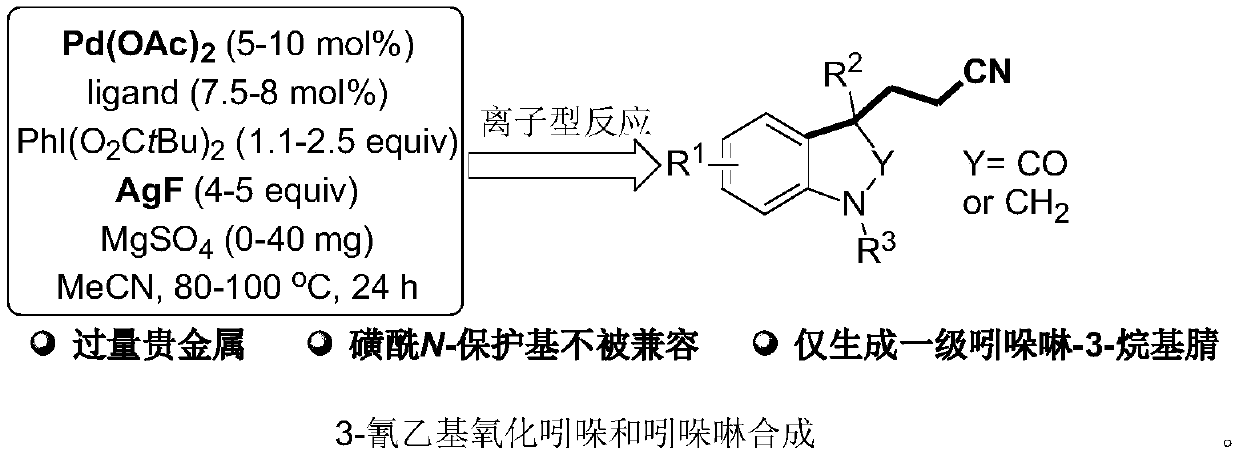
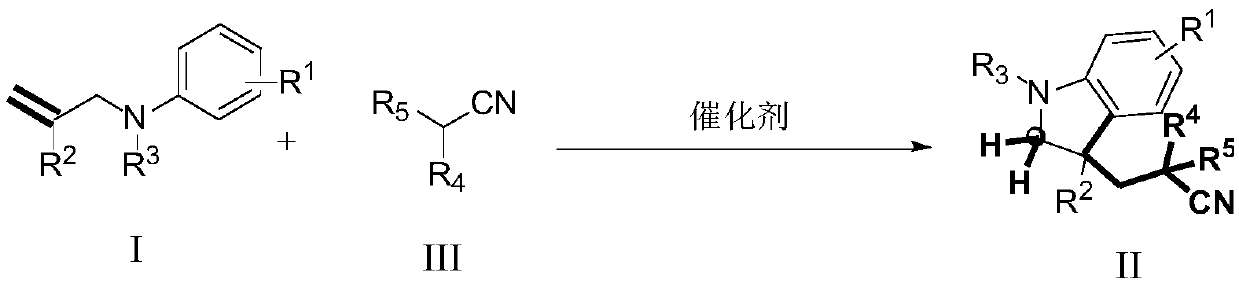
Method for synthesizing cyanoalkyl indoline by cyano-alkylation of N-allylaniline
A technology for synthesizing cyanoalkyl indolines and allylaniline cyanoanes, which is applied in organic chemistry, bulk chemical production, etc., can solve the problems of product limitation, limitation, and high cost, and achieve wide substrate range and high reaction efficiency. The effect of mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of non-activated olefins formula I compound (taking 1a2 synthesis as an example), the reaction scheme is as follows:
[0039]
[0040] Operation steps: Add p-toluidine (1.607g, 15.0mmol), CH 2 Cl 2 (50.0 mL), and Et 3N (4.170 mL, 30.0 mmol) and finally acetyl chloride (1.273 mL, 18.0 mmol) was added. The reaction mixture was stirred at room temperature with a magnetic stirrer. After TLC showed that the p-toluidine of the raw material was consumed, the 3 solution (100 mL), the reaction was quenched with CH 2 Cl 2 (100.0 mL) was extracted 3 times. The combined organic phases were washed twice with brine (50 mL). The solid obtained by distilling off the organic solvent was washed with a mixture of petroleum ether / ethyl acetate (5:1, volume ratio) to obtain p-methylacetanilide (2.104 g, 94% yield) as a white solid. To a stirred solution of p-methylacetanilide (1.492 g, 10.0 mmol) and NaOH (600 mg, 15.0 mmol) in DMF (30 mL) was added 2-methyl-3-bromoprop...
Embodiment 2
[0046] N-allyl aniline cyanoalkylation synthesis cyanoalkyl indoline (taking the synthesis of 2a1 as an example), the reaction scheme is as follows:
[0047]
[0048] Operation steps: Add N-allylaniline derivative 1a1 (57mg, 0.3mmol), catalyst DTBP (88mg, 0.6mmol) and acetonitrile solvent (3.0mL) to a Schlenk tube equipped with a magnetic stirrer under argon protection . The mixture was heated and stirred at 140°C for 6h, cooled with saturated Na 2 S 2 o 3 Quenched with aqueous solution (1.0 mL) and water (10.0 mL). The resulting mixture was extracted three times with dichloromethane (10.0 mL), and the organic phases were combined. The organic solvent was evaporated, and the residue was subjected to column chromatography using 300-400 mesh silica gel (petroleum ether and ethyl acetate as eluents) to obtain 3-cyanoalkylindoline product 2a1.
[0049] With reference to above-mentioned embodiment 2, investigate catalyst, the impact of temperature on reaction, specific situ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com