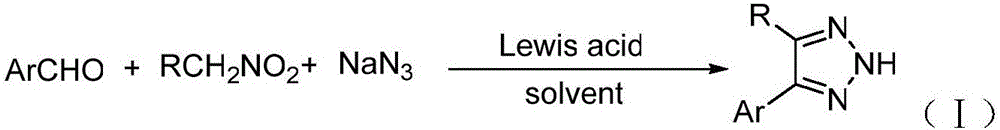Synthesis method of NH-1,2,3-triazole compound
A synthesis method and acid catalyst technology, applied in organic chemistry and other fields, can solve the problems of high toxicity of raw materials, high reaction temperature, and long reaction time, and achieve the effects of low catalyst, high reaction efficiency, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of 4-phenyl-2H-1,2,3-triazole:
[0023] The reaction formula is:
[0024]
[0025] The specific steps are: add 0.33mmol benzaldehyde, 0.50mmol nitromethane, 0.39mmol sodium azide, 0.03mmol AlCl to a 50mL round bottom flask 3 , 2mLDMSO, magnetically stirred at 80°C for 12 hours, then extracted the reaction solution with ethyl acetate, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain the crude product. Ethyl acetate / petroleum ether=1:5 (V / V) was used as the eluent for column separation and purification to obtain the desired product, which was a white solid with a yield of 94%.
[0026] The proton nuclear magnetic spectrogram result of gained product is: 1 HNMR (600MHz, DMSO-d 6 ): δ8.32(s,1H),7.84(d,J=7.4Hz,2H),7.43(t,J=7.7Hz,2H),7.33(t,J=7.4Hz,1H).
Embodiment 2
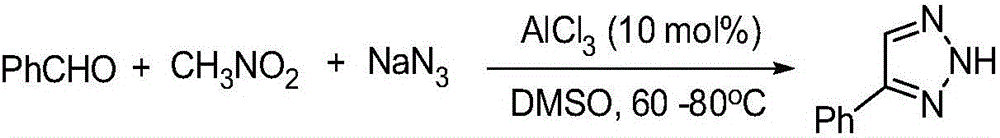
[0028] Synthesis of 4-phenyl-2H-1,2,3-triazole:
[0029] The reaction formula is:
[0030]
[0031] The specific steps are: add 0.33mmol benzaldehyde, 0.50mmol nitromethane, 0.39mmol sodium azide, 0.03mmol ZnCl to a 50mL round bottom flask 2 , 2mLDMSO, magnetically stirred at 80°C for 12 hours, then extracted the reaction solution with ethyl acetate, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain the crude product. Ethyl acetate / petroleum ether=1:5 (V / V) was used as the eluent for column separation and purification to obtain the desired product, which was a white solid with a yield of 75%.
[0032] The proton nuclear magnetic spectrogram result of gained product is: 1 HNMR (600MHz, DMSO-d 6 ): δ8.32(s,1H),7.84(d,J=7.4Hz,2H),7.43(t,J=7.7Hz,2H),7.33(t,J=7.4Hz,1H).
Embodiment 3
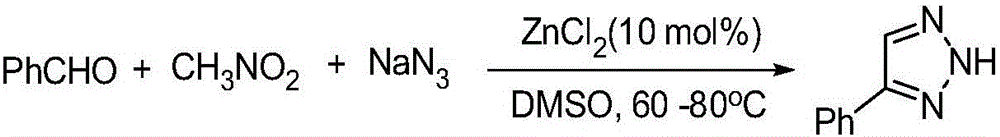
[0034] Synthesis of 4-phenyl-2H-1,2,3-triazole:
[0035] The reaction formula is:
[0036]
[0037] The specific steps are: add 0.33mmol benzaldehyde, 0.50mmol nitromethane, 0.39mmol sodium azide, 0.03mmol FeCl to a 50mL round bottom flask 3, 2mLDMSO, magnetically stirred and reacted at 80°C for 12 hours, then extracted the reaction solution with ethyl acetate, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain the crude product. Ethyl acetate / petroleum ether=1:5 (V / V) was used as the eluent for column separation and purification to obtain the desired product, which was a white solid with a yield of 70%.
[0038] The proton nuclear magnetic spectrogram result of gained product is: 1 HNMR (600MHz, DMSO-d 6 ): δ8.32(s,1H),7.84(d,J=7.4Hz,2H),7.43(t,J=7.7Hz,2H),7.33(t,J=7.4Hz,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com