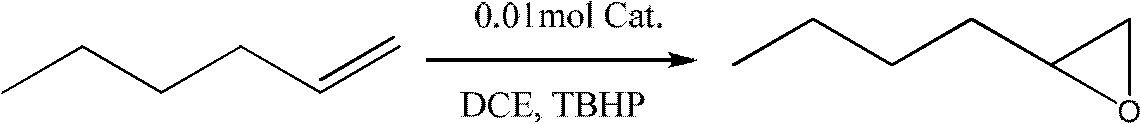Novel epoxidation catalyst, preparation method and applications
A technology for epoxidation and catalysts, which is applied in the field of preparation of new epoxidation catalysts, can solve the problems of unstable transition metal complexes, difficulty in catalyst recycling, difficult recovery of ligand synthesis, etc., and achieves strong substrate universality, Effect of constant conversion and selectivity, high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
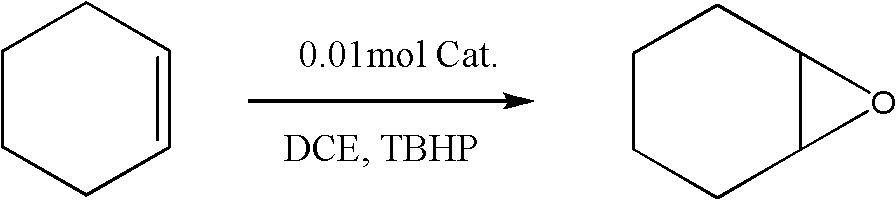
Examples
preparation example Construction
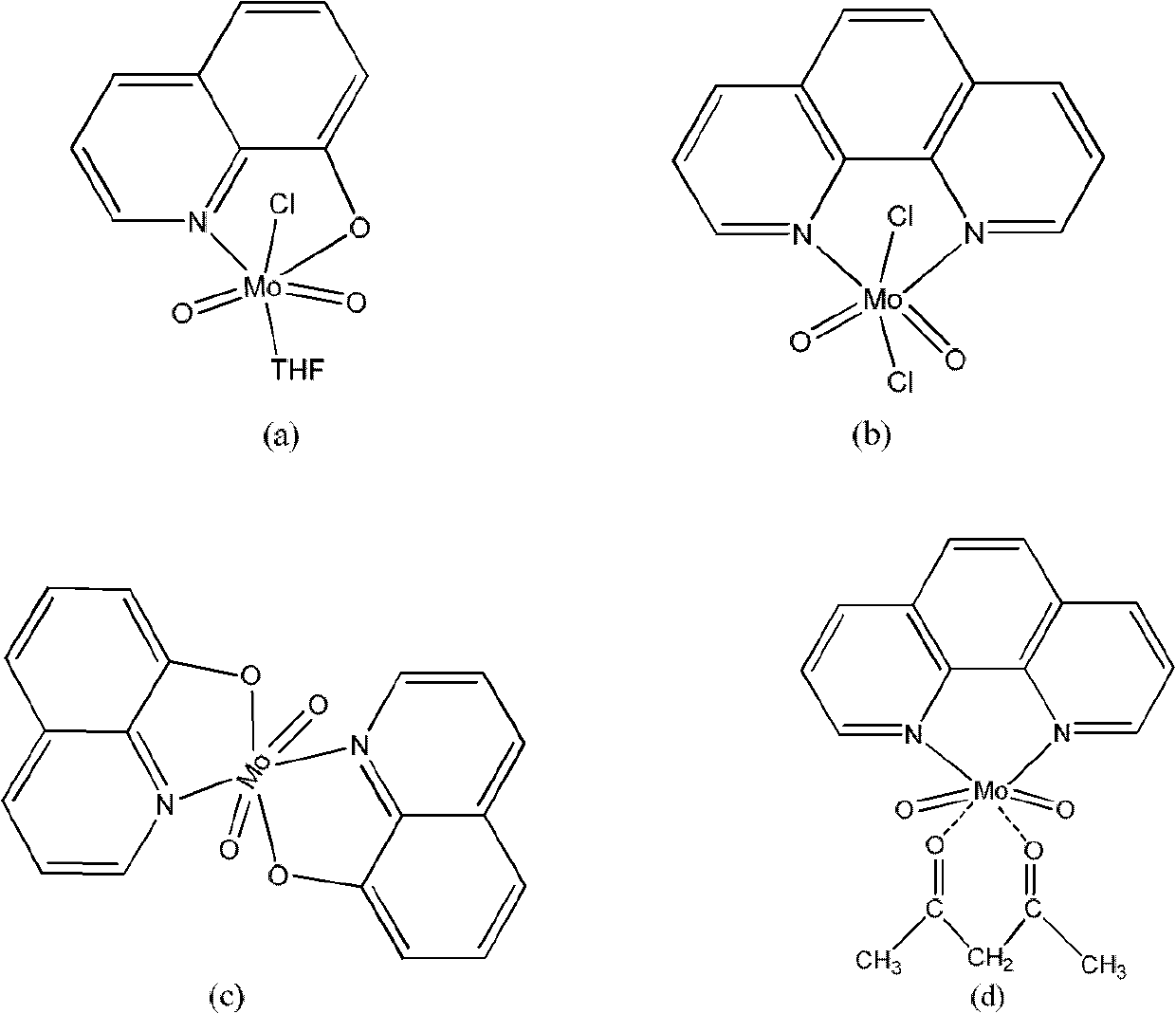
[0026] Preparation of molybdenum dioxide adduct:
[0027] ①According to the molar ratio, add 0.01 molar parts of sodium molybdate to 20 mL of concentrated hydrochloric acid, stir and dissolve at room temperature, extract three times with 300 parts of ether, dry overnight with anhydrous sodium sulfate, and remove the solvent by rotary evaporation to obtain dichlorodioxygen Molybdenum crystals, dissolved molybdenum dichloride crystals in tetrahydrofuran and rotary evaporation in the dark, to obtain flaky light yellow molybdenum dichloride and tetrahydrofuran solvent adduct MoO 2 Cl 2 (THF) 2 .
[0028] ② Dissolve 0.1 mole part of molybdenum trioxide in 30 mL of hydrochloric acid with a concentration of 6 mol / L under heating, add acetylacetone after cooling, and precipitate white crystals as molybdenum dioxide acetylacetonate adduct MoO 2 (acac) 2 .
Embodiment 1
[0030] The preparation of chlorotetrahydrofuran dioxy molybdenum 8-hydroxyquinoline salt (a): under stirring, the solvent adduct MoO 2 Cl 2 (THF) 2 (0.5mmol) in tetrahydrofuran (THF) 20mL was added to ligand 8-hydroxyquinoline (0.5mmol) in 20mL THF solution. The color of the solution immediately changed to yellow. After stirring at room temperature for 30 minutes, a straight yellow microcrystalline precipitate formed. The precipitate was separated, washed three times with n-hexane, and dried to obtain molybdenum dioxymolybdenum monochlorotetrahydrofuran 8-hydroxyquinoline salt complex (a) as a solid with a yield of 87%.
Embodiment 2
[0032] O-phenanthroline dichlorodioxymolybdenum complex (b) preparation: under stirring, the solvent adduct MoO 2 Cl 2 (THF) 2 (0.5mmol) of tetrahydrofuran (THF) solution 20mL was added in 20mL THF solution of ligand o-phenanthroline (0.5mmol), and the color of the solution immediately turned into light pink. After stirring at room temperature for 50 minutes, a pale pink precipitate formed. The precipitate was separated and washed three times with n-hexane to obtain pale pink microcrystalline complex (b) with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com