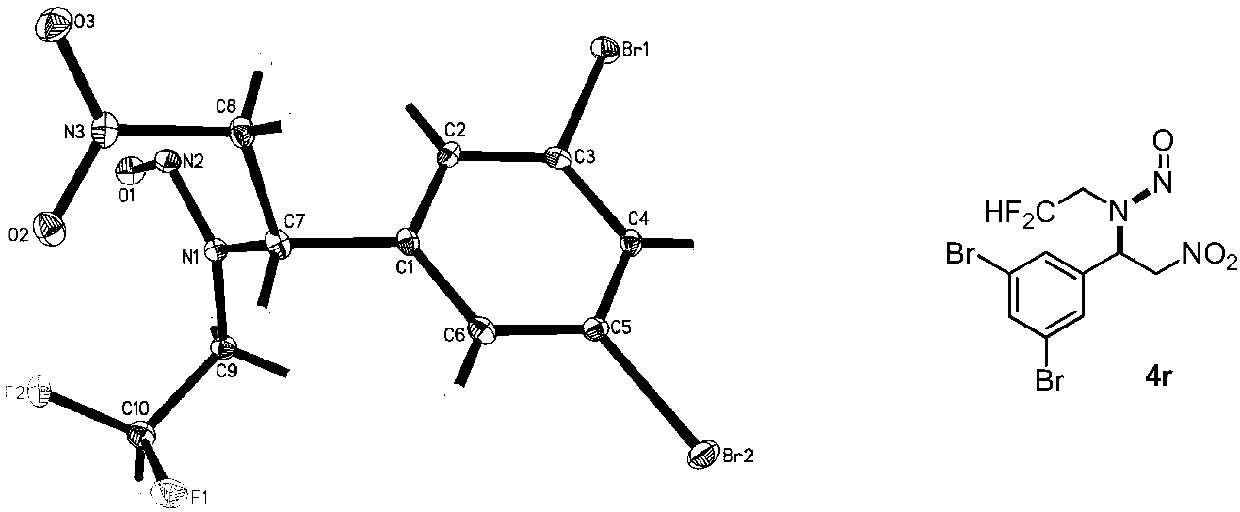
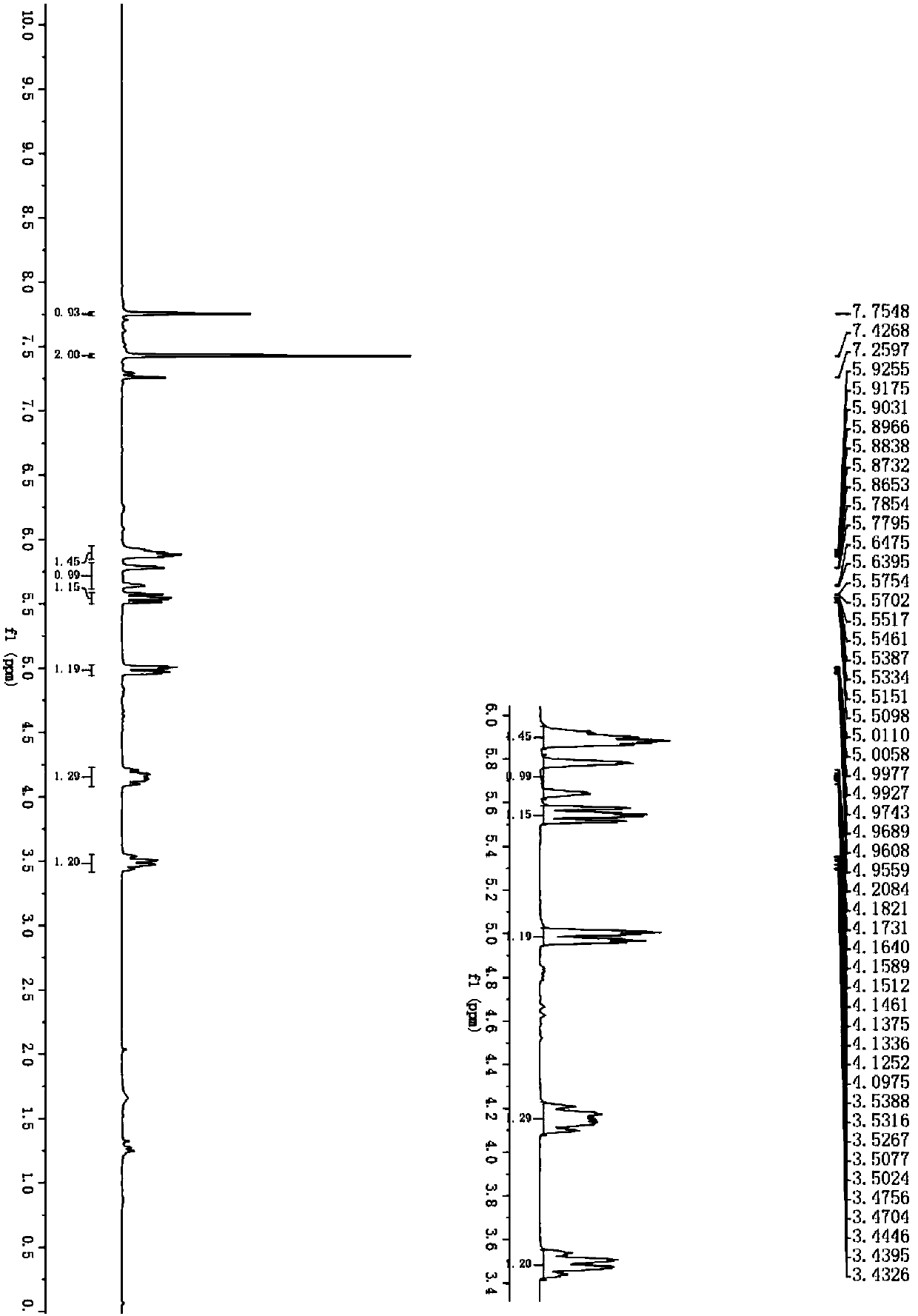
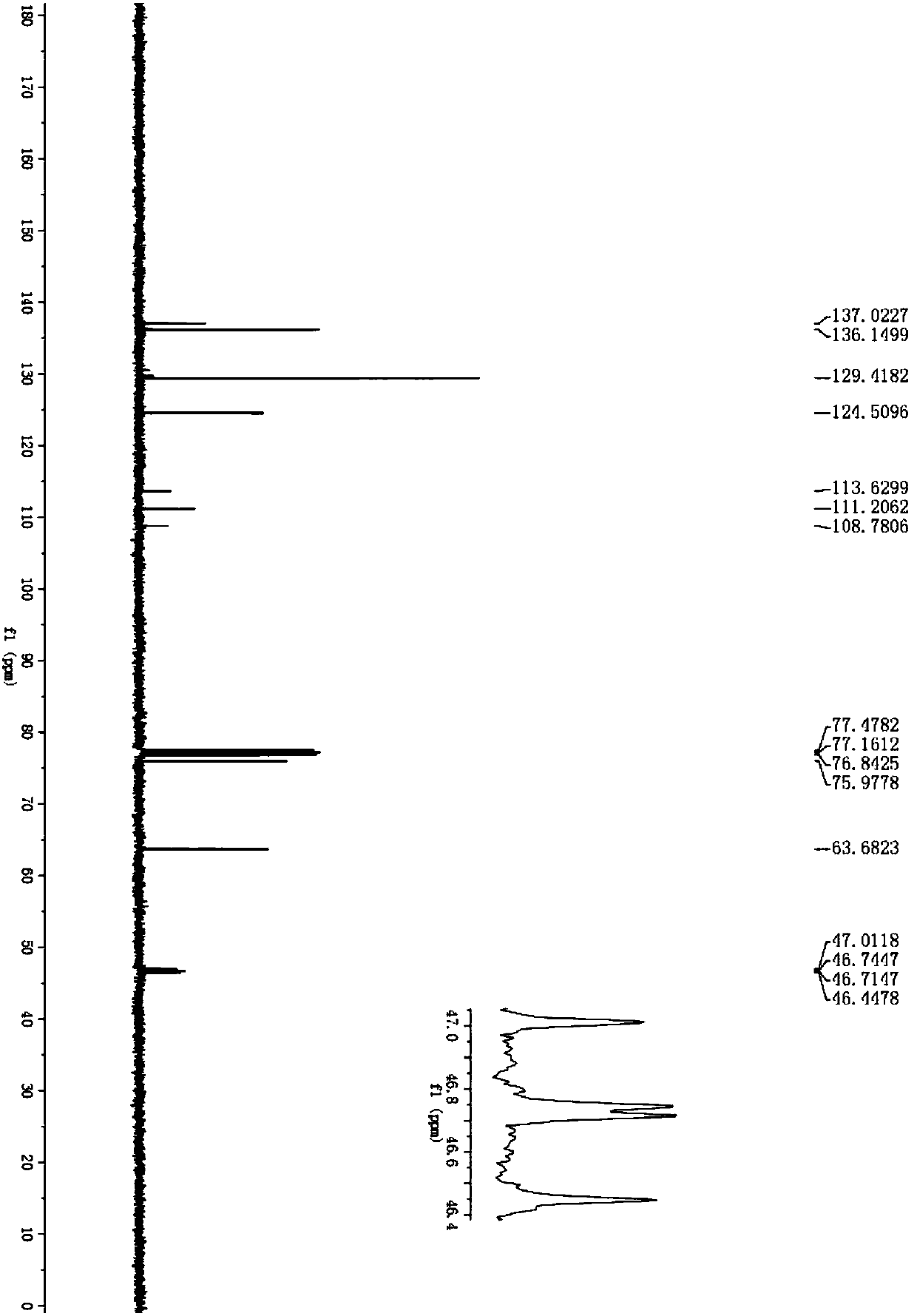
Difluoroethyl-containing nitrosamine compounds and preparation method thereof
A technology of difluoroethylnitrosamine and difluoroethylamine, which is applied in the field of chemistry, can solve problems that have not been reported, and achieve the effects of good substrate universality, easy product, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of Compound 4a
[0026]
[0027] Add difluoroethylamine (1,352.3μL, 5.0mmol), tert-butyl nitrite (2,1.9mL, 16.0mmol), 1-phenyl-2-nitroethylene shown in the formula (II) successively into a 5mL reaction flask (3a, 149.0 mg, 1.0 mmol) and acetic acid (2.9 μL, 0.05 mmol), stirred at room temperature for 6 hours. After the reaction was detected by thin-layer chromatography, it was separated by silica gel column chromatography (300-400 mesh) (petroleum ether: ethyl acetate = 5:1) to obtain 246.1 mg of a yellow oil with a yield of 95%. NMR and high-resolution Mass spectrometry analysis results are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.45–7.43(m,3H),7.32(t,J=3.4Hz,2H),6.01(dd,J=5.2,9.8Hz,1H),5.75(tt,J=4.0,55.8Hz,1H ),5.56(dd,J=9.8,18.0Hz,1H),4.99(dd,J=5.2,14.8Hz,1H),4.19–4.07(m,1H),3.40(qd,J=12.8,5.2Hz, 1H); 13 C NMR (100MHz, CDCl 3 )δ132.9, 130.2, 129.9, 127.5, 111.3 (t, J=242.2Hz, 1C), 76.1, 64.8, 35.8 (dd, J=26.8, 30.4Hz, 1C); 19 F NMR (376MHz, CDCl 3...
Embodiment 2
[0029] Synthesis of compound 4b
[0030]
[0031] Add difluoroethylamine (1,352.3μL, 5.0mmol), tert-butyl nitrite (2,1.9mL, 16.0mmol), 1-(2-methoxyphenyl )-2-nitroethylene (3b, 179.2 mg, 1.0 mmol) and acetic acid (2.9 μL, 0.05 mmol), stirred at room temperature for 6 hours. After the reaction was detected by thin-layer chromatography, it was separated by silica gel column chromatography (300-400 mesh) (petroleum ether: ethyl acetate = 5:1) to obtain 260.2 mg of a yellow solid with a yield of 90% and a melting point of 76.9-78.6°C. NMR and high-resolution mass spectrometry test analysis results are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.40(t, J=7.8Hz, 1H), 7.07(d, J=7.8Hz, 1H), 7.01–6.98(m, 2H), 6.38(dd, J=4.4, 10.2Hz, 1H), 5.77(tdd, J=3.0, 4.8, 55.6Hz, 1H), 5.54(dd, J=10.2, 14.8Hz, 1H), 4.96(dd, J=4.4, 14.8Hz, 1H), 4.22–4.11(m, 1H), 3.90(s, 3H), 3.50–3.39(m, 1H); 13 C NMR (100MHz, CDCl 3 )δ156.9, 131.4, 128.0, 121.5, 120.9, 111.5, 111.3 (t, J = 243.6Hz, 1C), 74.7, 59...
Embodiment 3
[0033] Synthesis of compound 4c
[0034]
[0035] Add difluoroethylamine (1,352.3 μL, 5.0 mmol), tert-butyl nitrite (2, 1.9 mL, 16.0 mmol), 1-(2-fluorophenyl)- 2-Nitroethylene (3c, 167.0 mg, 1.0 mmol) and acetic acid (2.9 μL, 0.05 mmol) were stirred at room temperature for 6 hours. After the reaction was detected by thin-layer chromatography, it was separated by silica gel column chromatography (300-400 mesh) (petroleum ether: ethyl acetate = 5:1) to obtain 274.3 mg of a yellow oil with a yield of 99%. NMR and high-resolution Mass spectrometry analysis results are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.48–7.42(m,1H),7.28–7.23(m,2H),7.21–7.16(m,1H),6.32(dd,J=4.8,9.8Hz,1H),5.91–5.75(m, 1H), 5.60(dd, J=9.8, 14.8Hz, 1H), 5.02(dd, J=4.8, 14.8Hz, 1H), 4.16–4.04(m, 1H), 3.61–3.49(m, 1H); 13 C NMR (100MHz, CDCl 3 )δ160.39(d, J=249.2Hz, 1C), 132.19(d, J=8.5Hz, 1C), 128.75(d, J=2.6Hz, 1C), 125.59(d, J=3.7Hz, 1C) ,120.38(d,J=13.2Hz,1C),116.76(d,J=21.4Hz,1C),111.17(t,J=243.7Hz,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com