Method for synthesizing 3-aryl thiopropionamide derivative
A technology of arylthiopropionamide and arylpropyne derivatives, which is applied in the field of preparation of 3-arylthiopropionamide derivatives, can solve few problems such as thioamides, and achieve universal application of strong substrates Sexuality, development promotion and emission reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
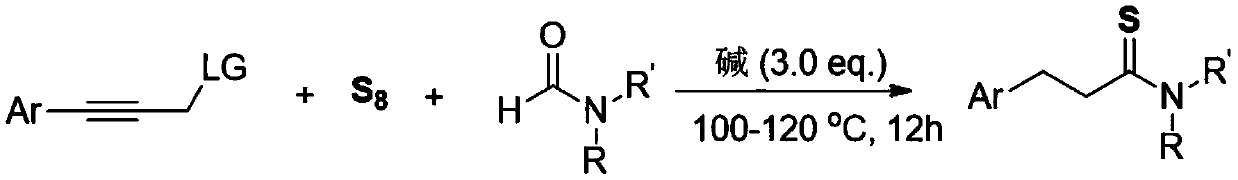
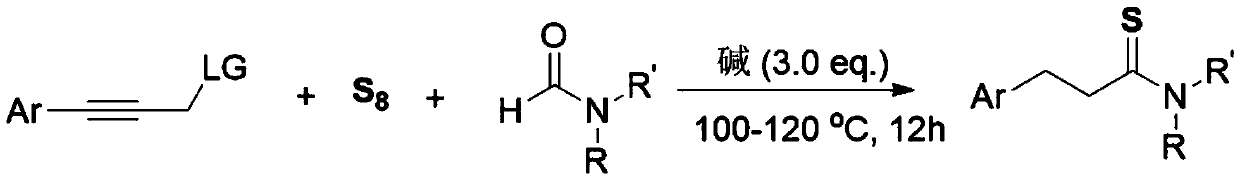
[0014] The invention discloses a three-component one-pot method for synthesizing 3-arylthiopropionamide derivatives of aryl propyne derivatives, elemental sulfur and formamide, comprising the following steps: aryl propyne substrate, Alkali is used as a promoter, and elemental sulfur is used as a sulfur source. In the solvent formamide, at 100-120 ° C, the reaction is stirred for 12 hours. The chemical reaction formula is as follows:
[0015]
[0016] The -Ar is phenyl, 4-methylphenyl, 3-methylphenyl, 2-methylphenyl, 4-ethylphenyl, 4-tert-butylphenyl, 4-methoxybenzene Base, 3-methoxyphenyl, 3-fluorophenyl, 3-chlorophenyl, 3-bromophenyl, 4-trifluoromethylphenyl, 4-phenylphenyl, 1-naphthyl, 2 - a kind of in thienyl;
[0017] The -R, -R' is one of hydrogen, methyl, ethyl, piperidinyl, and morpholinyl;
[0018] The -LG is one of N-methyl, N-methylphenyl, N,N-dimethyl, piperidinamine, morpholinamine, hydroxyl, phenoxy, phenylmercapto and chlorine.
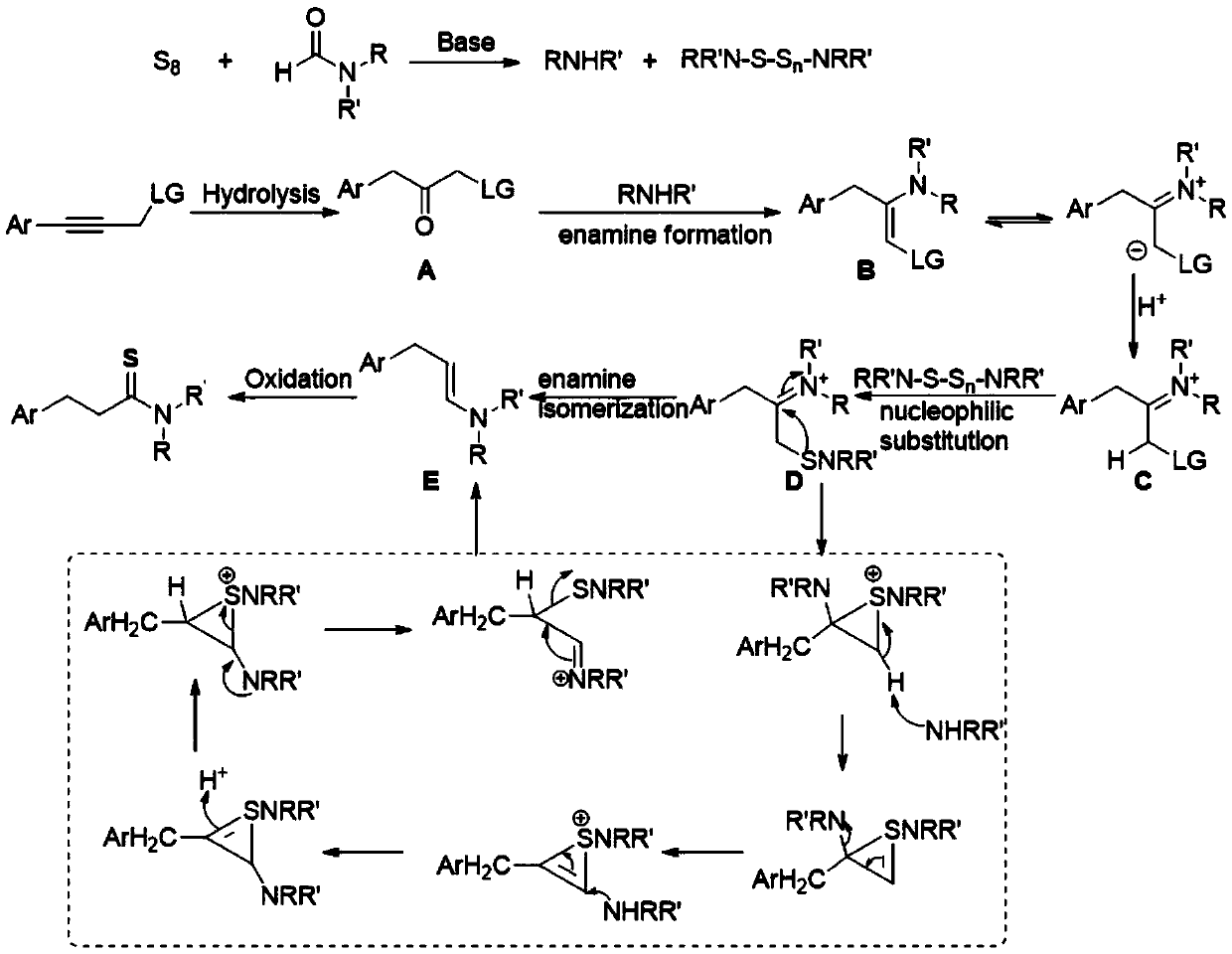
[0019] After the reaction w...
specific Embodiment 1
[0020] Specific embodiment one: with 41.4 milligrams (0.2mmol) N-(3-phenylprop-2-yn-1-yl)aniline, 61.4 milligrams (0.24mmol) elemental sulfur, 34.8 milligrams (3 equivalents) potassium fluoride, Add 2 mL of solvent N,N-dimethylformamide. The reaction was stirred at 120°C for 12 hours. After the reaction was cooled, the mixture was poured into ethyl acetate and washed with brine (2×15 mL). After extracting the aqueous layer with ethyl acetate, the combined organic layers were washed with anhydrous Na 2 SO 4 Dry, filter, and use a rotary evaporator to remove the solvent from the filtrate to obtain a residue, conduct column separation on the residue through a silica gel column, and rinse with the eluent, collect the effluent containing the target product, combine the effluent and vacuum Concentrate to remove the solvent to obtain the target product. The residue was washed through a silica gel column with an eluent prepared with petroleum ether and ethyl acetate at a volume ra...
specific Embodiment 2
[0021] Specific embodiment two: 44.2 milligrams (0.2mmol) N-(3-(p-tolyl) prop-2-yn-1-yl) aniline, 61.4 milligrams (0.24mmol) elemental sulfur, 34.8 milligrams (3 equivalents) fluorine Potassium chloride was added to 2 ml of solvent N,N-dimethylformamide. The reaction was stirred at 120°C for 12 hours. After the reaction was cooled, the mixture was poured into ethyl acetate and washed with brine (2×15 mL). After extracting the aqueous layer with ethyl acetate, the combined organic layers were washed with anhydrous Na 2 SO 4 Dry, filter, and use a rotary evaporator to remove the solvent from the filtrate to obtain a residue, conduct column separation on the residue through a silica gel column, and rinse with the eluent, collect the effluent containing the target product, combine the effluent and vacuum Concentrate to remove the solvent to obtain the target product. The residue was washed through a silica gel column with an eluent prepared with petroleum ether and ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com