Method for synthesizing 2,5-furandimethanol by selective hydrogenation of 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and furandimethanol, which is applied in the field of selective hydrogenation of 5-hydroxymethylfurfural to synthesize 2,5-furandimethanol, which can solve the problem of low conversion rate of substrate and product yield, limitations of catalytic efficiency and other issues, to achieve good application value and industrialization prospects, multiple acid-base sites, and easy separation and recovery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of Magnetic Metal-Organic Coordination Polymer
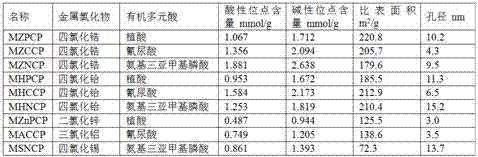
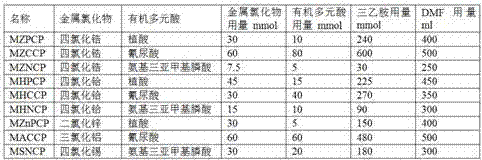
[0041] Add nanometer iron ferric oxide into 400mL dimethylformamide solution containing 30mmol metal chloride according to the metal chloride / iron molar ratio of 2:1, and stir for 30min with the assistance of ultrasound; Slowly add 400mL of dimethylformamide solution containing 10mmol of organic polybasic acid and 240mmol of triethylamine dropwise at 5mL / min and 1mL / min respectively; continue to stir at room temperature for 4h, raise the temperature to 70°C, and then stand and age for 4h ;Separate the solid precipitate with the help of a magnet, and wash the precipitate repeatedly with ethanol and ether until no chloride ions are detected; dry the washed solid precipitate at 80°C for 12 hours in vacuum, and grind it to about 120 meshes. A magnetic metal organic coordination polymer catalyst is obtained.
[0042] The amount of raw materials and the types of reactants when using different metal chloride...
Embodiment 2
[0047] The above-mentioned magnetic metal-organic coordination polymer catalyst is used to carry out selective hydrogenation of 5-hydroxymethylfurfural to synthesize 2,5-furandimethanol. The synthesis method is:
[0048] Add 20g of isopropanol, 0.4g of 5-hydroxymethylfurfural and 0.16g of magnetic metal-organic coordination polymer catalyst in a 50mL reactor, seal and replace the air in the kettle with nitrogen continuously for 6 times; at a stirring speed of 400 rpm Raise the temperature to 130°C and perform selective hydrogenation reaction for 8 hours to obtain 2,5-furandimethanol.
[0049] In order to investigate the reusability of the catalyst, after the reaction was completed, an external magnet was added to separate MZPCP from the reaction solution, and then it was washed and dried to carry out the next selective hydrogenation reaction according to the above reaction conditions.
[0050] As compared with the catalyst provided by the invention and the hydrogenation synth...
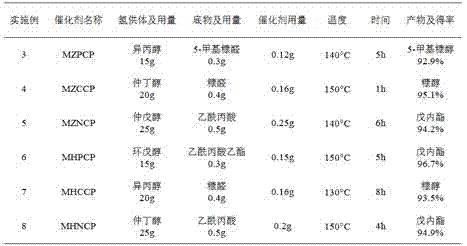
Embodiment 3~8
[0054] Examples 3-8 Catalysts in the selective hydrogenation conversion of other carbonyl compounds
[0055] Add low-carbon alcohols, carbonyl-containing compounds and magnetic metal-organic coordination polymer catalysts into a 50mL reactor, and after sealing, replace the air in the reactor with nitrogen continuously for 6 times; heat up at a stirring speed of 400 rpm, and the selective hydrogenation reaction is The corresponding products can be obtained.
[0056] Among them, the reaction substrates, catalysts and reaction conditions used are summarized as follows:
[0057]
[0058] It can be seen from the above table that the catalyst provided by the present invention is also suitable for the selective hydrogenation reaction of other carbonyl compounds, and has a better reaction yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com