Method for synthesizing sulfone compounds under photocatalysis condition
A photocatalytic and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of limited universality of substrates and harsh reaction conditions , to achieve the effect of broadening new applications, low prices, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
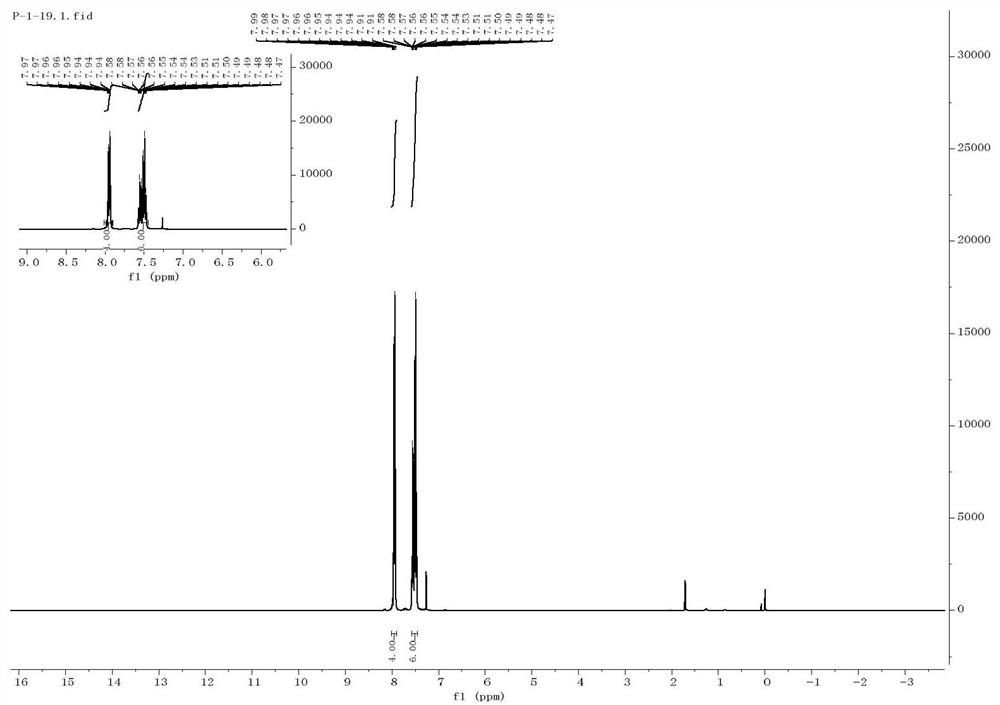
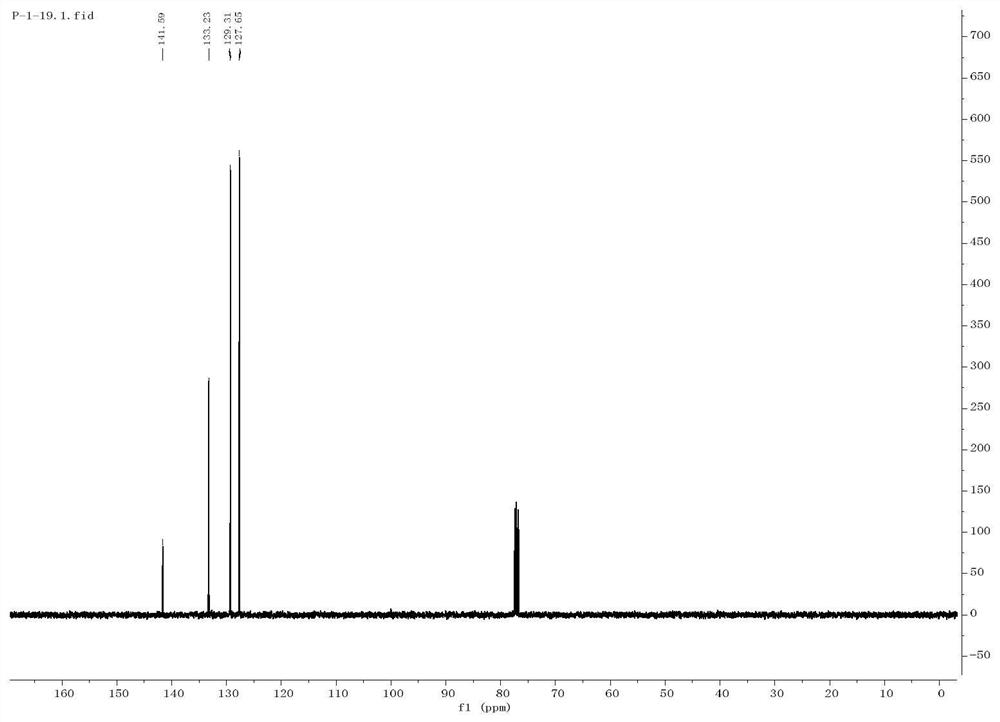
[0039] The preparation of embodiment 1 diphenylsulfone
[0040] Get a quartz reaction tube, add a magnetic stirrer thereto, then add 0.3mmol of phenylhydrazine, 0.9mmol of sodium benzenesulfinate, 0.9mmol of cesium carbonate (Cs2 CO 3 ), 5mmol% photosensitizer polyacid salt ([(n-Bu 4 )N] 4 [W 10 o 32 ], TBADT), and finally 1 mL of dimethyl sulfoxide (DMSO) was added.
[0041] Then add a three-way gas guide head with a balloon above the quartz reaction tube, first use liquid nitrogen to freeze the reaction stock solution completely, then use an oil pump to evacuate the quartz reaction tube, and then fill the balloon with oxygen; stir with a magnetic stirrer Under the condition of oxygen, the reaction was carried out under the irradiation of blue LED light for 12 hours, the final product was detected by TLC, and finally the final product diphenyl sulfoxide was obtained by separation by column chromatography with a yield of 58%.
[0042] The reaction equation is as follows: ...
Embodiment 2
[0046] The preparation of embodiment 2 4-methyldiphenyl sulfone
[0047] Get a quartz reaction tube, add a magnetic stirrer thereto, then add 0.3mmol of 4-methylphenylhydrazine, 0.9mmol of sodium benzenesulfinate, 0.9mmol of cesium carbonate (Cs 2 CO 3 ), 5 mmol% photosensitizer Eosin Y, and finally 1 mL of dimethyl sulfoxide (DMSO) was added.
[0048] Then add a three-way gas guide head with a balloon above the quartz reaction tube, first use liquid nitrogen to freeze the reaction stock solution completely, then use an oil pump to evacuate the quartz reaction tube, and then fill the balloon with oxygen; stir with a magnetic stirrer Under the condition of oxygen, the reaction was carried out under the irradiation of blue LED light for 12 h, the final product was detected by TLC, and finally the final product 4-methyldiphenyl sulfone was obtained by separation by column chromatography with a yield of 52%.
[0049] The reaction equation is as follows:
[0050]
[0051] 4-M...
Embodiment 3
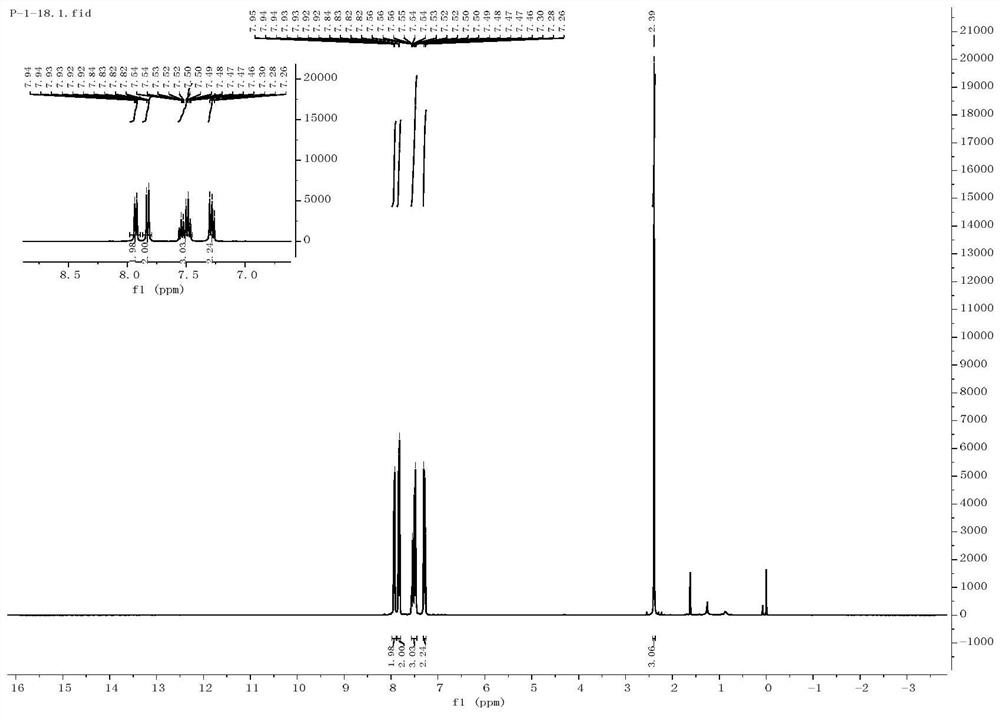
[0052] The preparation of embodiment 3 4-cyanodiphenyl sulfone
[0053] Get a quartz reaction tube, add a magnetic stirrer thereto, then add 0.3mmol of 4-cyanophenylhydrazine, 0.9mmol of sodium benzenesulfinate, 0.9mmol of cesium carbonate (Cs 2 CO 3 ), 15mmol% photosensitizer polyacid salt ([(n-Bu 4 )N] 4 [W 10 o 32 ], TBADT), and finally 1 mL of dimethyl sulfoxide (DMSO) was added.
[0054] Then add a three-way gas guide head with a balloon above the quartz reaction tube, first use liquid nitrogen to freeze the reaction stock solution completely, then use an oil pump to evacuate the quartz reaction tube, and then fill the balloon with oxygen; stir with a magnetic stirrer Under the condition of oxygen, the reaction was carried out under the irradiation of blue LED light for 12 h, the final product was detected by TLC, and finally the final product 4-cyanodiphenyl sulfone was obtained by separation by column chromatography with a yield of 87%.
[0055] The reaction equat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com