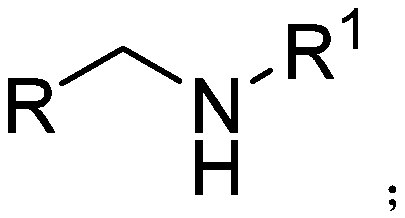
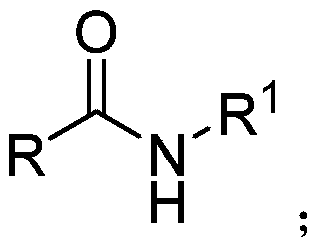
Secondary amine derivative synthesized through rare earth catalysis, and preparation method thereof
A rare earth catalysis and derivative technology, which is applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, chemical instruments and methods, etc. The effect of ensuring operator health, low cost and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
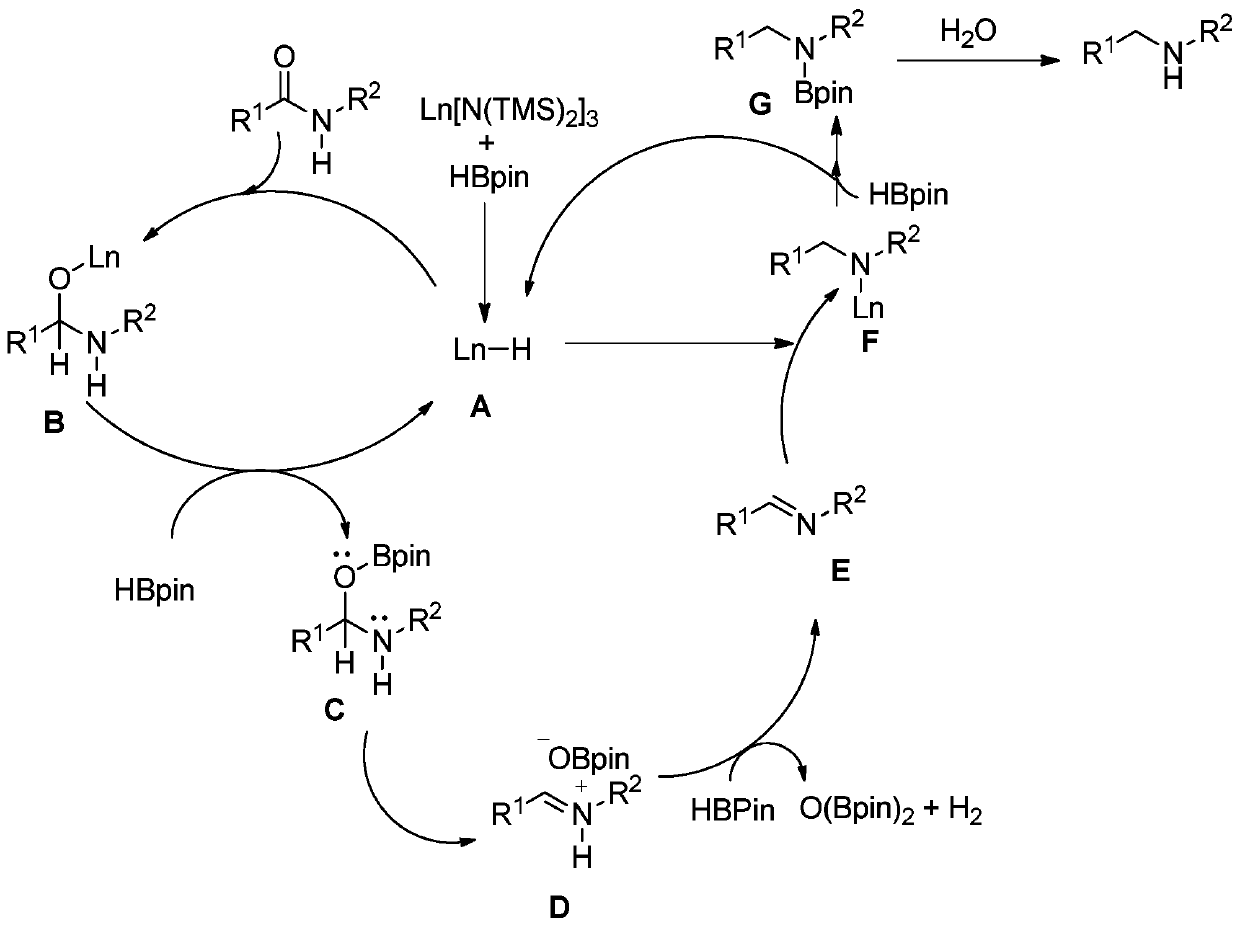
Method used
Image
Examples
Embodiment 1
[0030] Under nitrogen protection, add raw material N-benzyl benzamide (0.5mmol), pinacol borane (3.0mmol), rare earth catalyst bistrimethylsilylamide yttrium (10mol%) and solvent toluene in the reaction vessel (3ml) were stirred and mixed; after mixing evenly, reacted at a temperature of 120° C. for 24 hours to obtain dibenzylamine; the yield of the final product was 90%.
[0031] Characterization data: 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.43-7.38(m,8H),7.35-7.31(m,2H),3.88(s,4H),1.75(brs,1H). 13 C NMR (CDCl 3 ,125MHz,ppm): δ140.5,128.5,128.2,127.0,53.5.
[0032] The structural formula of dibenzylamine is as follows:
[0033]
[0034] Its reaction formula is
[0035]
Embodiment 2
[0037] Under nitrogen protection, in reaction vessel, add raw material N-benzyl-4-methoxybenzamide (0.5mmol), pinacol borane (3.5mmol), rare earth catalyst bistrimethylsilylamino yttrium ( 9mol%) and solvent toluene (3ml) are stirred and mixed; After mixing uniformly, react 25h under the condition of 110 ℃ in temperature, make N-benzyl-4-methoxyphenylmethylamine; Final product yield is 89%.
[0038] Characterization data: 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.37-7.34(m,4H),7.30-7.28(m,3H),6.92-6.89(m,2H),3.82(s,5H),3.78(s,2H),1.85 (brs,1H). 13 C NMR (CDCl 3 ,125MHz,ppm):δ158.8,140.5,132.6,129.4,128.5,128.2,127.0,113.9,55.3,53.2,52.7.
[0039] The structural formula of N-benzyl-4-methoxyphenylmethylamine is
[0040]
[0041] Its reaction formula is
[0042]
Embodiment 3
[0044] Under the protection of nitrogen, add raw materials N-benzyl-2-furan carboxamide (0.5mmol), pinacol borane (2.5mmol), rare earth catalyst bistrimethylsilylamino yttrium (12mol%) to the reaction vessel Stir and mix with the solvent toluene (3ml); after mixing evenly, react at a temperature of 100°C for 22h to obtain N-benzyl-1-(2-furan)-methylamine; the yield of the final product is 85% .
[0045] Characterization data: 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.39-7.38(m,1H),7.35-7.34(m,4H),7.29-7.26(m,1H),6.34(dd,J=1.9Hz,J=3.1Hz,1H ), 6.20(d, J=3.1Hz, 1H), 3.80(s, 4H), 1.97(brs, 1H). 13 C NMR (CDCl 3 ,125MHz,ppm):δ154.0,141.9,140.0,128.5,128.3,127.1,110.2,107.1,52.9,45.5.
[0046]The structural formula of N-benzyl-1-(2-furan)-methylamine is as follows:
[0047]
[0048] Its reaction formula is
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com