Patents
Literature
87 results about "Achirality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for the synthesis of phosphorus atom modified nucleic acids
Described herein are methods of syntheses of phosphorous atom-modified nucleic acids comprising chiral X-phosphonate moieties. The methods described herein provide backbone-modified nucleic acids in high diasteteomeric purity via an asymmetric reaction of an achiral molecule comprising a chemically stable H-phophonate moiety with a nucleoside / nucleotide.
Owner:WAVE LIFE SCI LTD
Method for the synthesis of phosphorus atom modified nucleic acids
Described herein are methods of syntheses of phosphorous atom-modified nucleic acids comprising chiral X-phosphonate moieties. The methods described herein provide backbone-modified nucleic acids in high diasteteomeric purity via an asymmetric reaction of an achiral molecule comprising a chemically stable H-phophonate moiety with a nucleoside / nucleotide.
Owner:WAVE LIFE SCI LTD
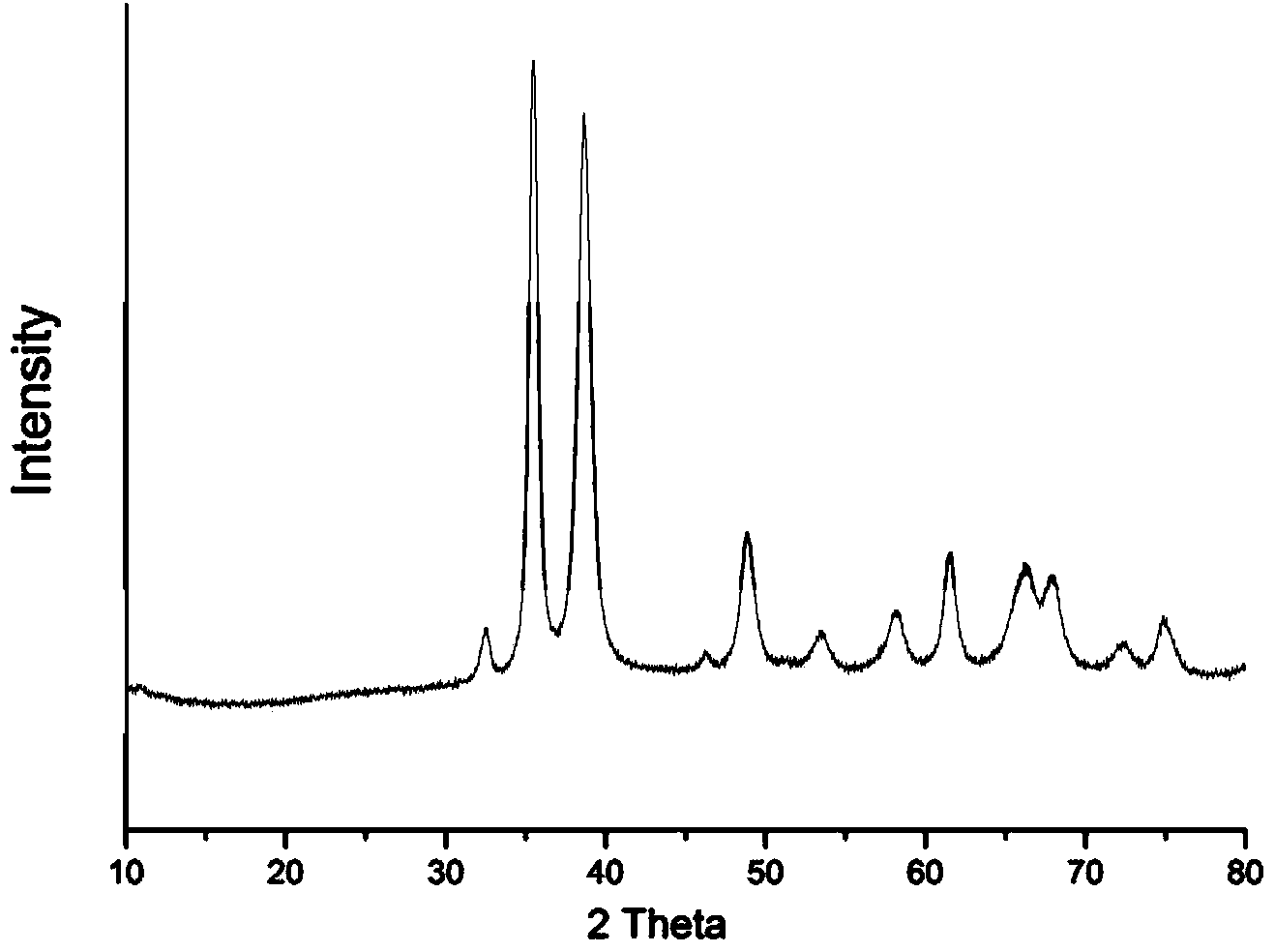
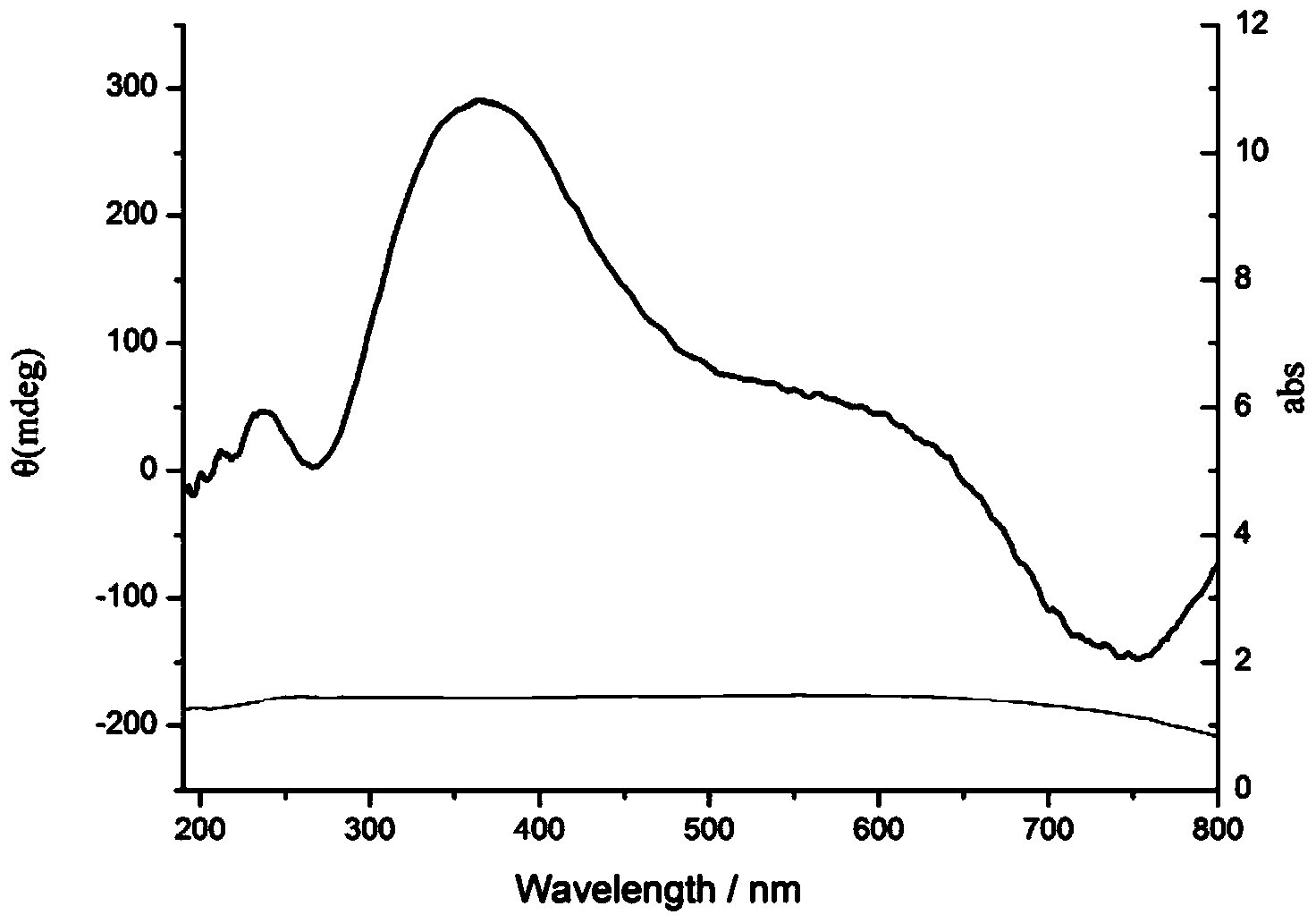
Preparation method of chiral nanometer copper oxide with optical activity
InactiveCN103864134AHigh optical activityEnhance amphipathicMaterial nanotechnologyCopper oxides/halidesRoom temperatureCopper oxide
The invention discloses a preparation method of chiral nanometer copper oxide with optical activity. The preparation method comprises the following steps of: (1) dissolving an achiral anionic surface-active agent and a chiral small molecule into water at room temperature to obtain an achiral anionic surface-active agent and chiral small molecule water solution; (2) adding inorganic copper salt to the achiral anionic surface-active agent and chiral small molecule water solution, and stirring to react for 10-60 minutes; (3) adding alkali to reaction liquid obtained from the step (2), then increasing temperature to 100-200 DEG C, and reacting for 30 minutes-6 hours; (4) after the reaction is finished, centrifugalizing or filtering the reaction liquid obtained from the step (3), and washing and drying to obtain the chiral nanometer copper oxide with the optical activity. The prepared chiral nanometer copper oxide with the optical activity shows very uniform flower-shaped appearance and shows outstanding optical activity by having chirality.
Owner:SHANGHAI JIAO TONG UNIV
Efficient process to induce enantioselectivity in procarbonyl compounds
ActiveUS20120264933A1High enantioselectivityImprove production yieldSilicon organic compoundsAmino preparation from aminesEnantio selectivityCoordination complex
The present invention provides an efficient method to induce the enantioselectivity in procarbonyl compounds using chiral organometallic complexes. The present invention also provides a method for producing chiral organometallic complexes using a chiral additive, achiral additive, a base and a metal salt.
Owner:LAURUS LABS
Inorganic mesoporous materials with chiral nematic structures and preparation method thereof
The present invention describes a composition and a method for producing mesoporous silica materials with a chiral organization. In the method, a polymerizable inorganic monomer is reacted in the presence of nanocrystalline cellulose (NCC) to give a material of inorganic solid with cellulose nanocrystallites embedded in a chiral nematic organization. The NCC can be removed to give a stable porous structure that retains the chiral organization of the NCC template. The new materials may be obtained as iridescent free-standing films with high surface area. Through control of the reaction conditions, the colour of the films can be varied across the entire visible spectrum. These are the first materials to combine mesoporosity with long-range chiral ordering that leads to photonic properties. Examples of possible applications of the materials are: lightweight reinforcement materials, low k dielectric materials, tunable reflective filters, adsorbents, stationary phases for chromatography of chiral or achiral substances, supports for catalysts (e.g., for asymmetric synthetic transformations), and as a template to generate other new porous materials (e.g., porous carbon or porous metals), preferably with chiral nematic structures.
Owner:FPINNOVATIONS INC
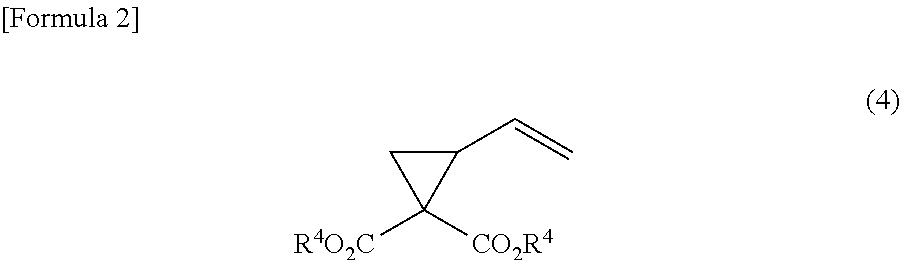
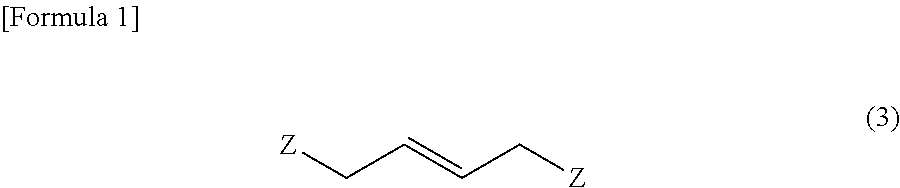
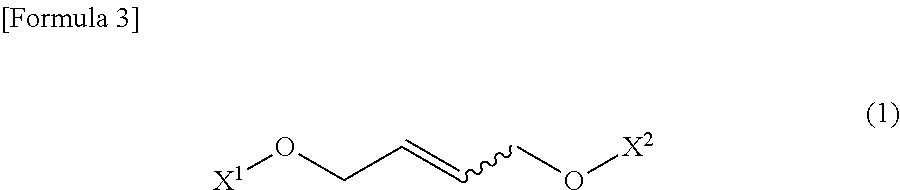
Method for producing 1-amino-1-alkoxycarbonyl-2-vinylcyclopropane
ActiveUS20130096339A1Easily produceCarbamic acid derivatives preparationOrganic compound preparationCarbonyl groupLeaving group
It is an object of the present invention to provide a novel method for producing (1R,2S) / (1S,2R)-1-amino-1-alkoxycarbonyl-2-vinylcyclopropane which is useful as a synthetic intermediate of therapeutic agents for hepatitis C and a synthetic intermediate thereof. According to the present invention, when a trans-2-butene derivative having a leaving group at each of the 1- and 4-positions is reacted with a malonic ester in the presence of a base, a specific amount of an alkali metal alkoxide or an alkali metal hydride is used as the base, and further a specific amount of a malonic ester is used to produce a cyclopropane diester, and further, chiral or achiral 1-amino-1-alkoxy-carbonyl-2-vinylcyclopropane and a salt thereof are synthesized using the cyclopropane diester.
Owner:API CORP (JP)
Optically isotropic liquid crystal medium and optical device
InactiveUS20130135544A1Heat stableStable lightingLiquid crystal compositionsStatic indicating devicesDopantOrganic chemistry
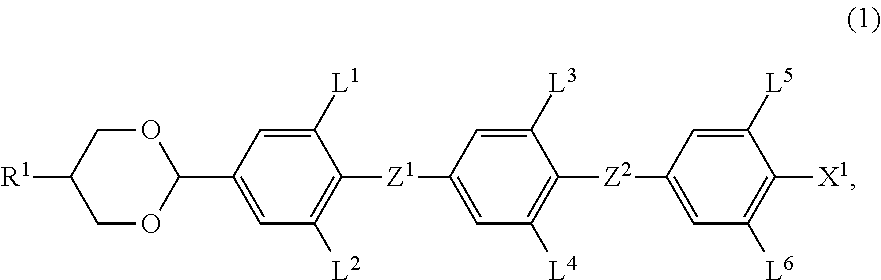
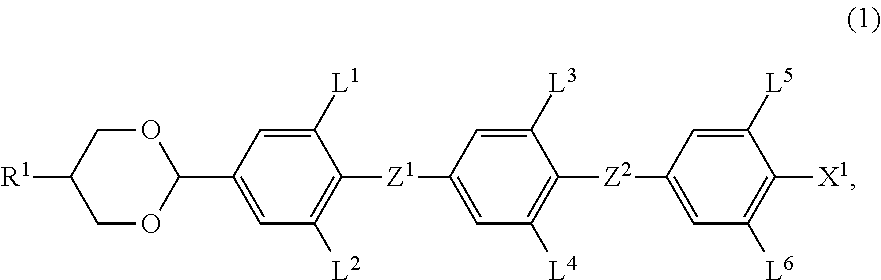
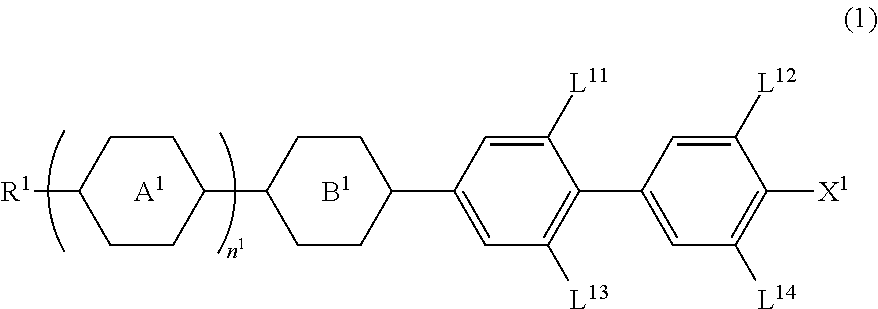
A liquid crystal composition is described, which exhibits an optically isotropic liquid crystal phase and contains an achiral component T and a chiral dopant. The achiral component T contains, as its first component, at least one compound selected from compounds represented by formula (1) in an amount of 32 wt % to 85 wt %,wherein R1 is alkyl, for example; L1, L2, L3, L4, L5 and L6 are each independently hydrogen or fluorine; Z1 and Z2 are each independently a single bond or —CF2O—, with at least one of Z1 and Z2 being —CF2O—; and X1 is halogen, for example.
Owner:JNC CORP +1
Process for the preparation of substituted pyrrolidine derivatives and intermediates
ActiveUS20060128789A1Easy to operateHigh yieldBiocideOrganic chemistryCombinatorial chemistryPyrrolidine
Two syntheses are provided; one for the preparation of (3R,4R)-4-(hydroxymethyl)pyrrolidin-3-ol, and other for the preparation of (3S,4R)-4-(hydroxymethyl)pyrrolidin-3-ol. (3R,4R)-4-(hydroxymethyl)pyrrolidin-3-ol is prepared using the achiral ylide prepared from benzylamine instead of phenethylamine (Scheme 3) which provides a crystalline intermediate. The synthesis of (3S,4R)-4-(hydroxymethyl)pyrrolidin-3-ol is achieved from (S)-diethylmalate as described in Scheme 4. A process for preparing camphor sultam is also provided.
Owner:BIOCRYST PHARM INC
Circular polarization luminescent material and preparation method and application thereof
ActiveCN111944515ASimple manufacturing methodMild conditionsMaterial nanotechnologyOrganic chemistryAchiralityLuminescent material
The invention discloses a method for preparing a circular polarization luminescent material. The preparation method comprises the following steps: (1) mixing a chiral substance with an achiral luminescent material, adding a solvent, and mixing again to obtain a mixed solution; (2) mixing the mixed solution obtained in the step (1) with grinding balls, and grinding the solution by adopting a wet ball-milling method to obtain the circular polarization luminescent material. According to the invention, the achiral luminescent material has the property of circular polarization luminescence, the combination of the luminescent material and chirality is realized, the method is simple, and the operability is strong.
Owner:INST OF CHEM CHINESE ACAD OF SCI
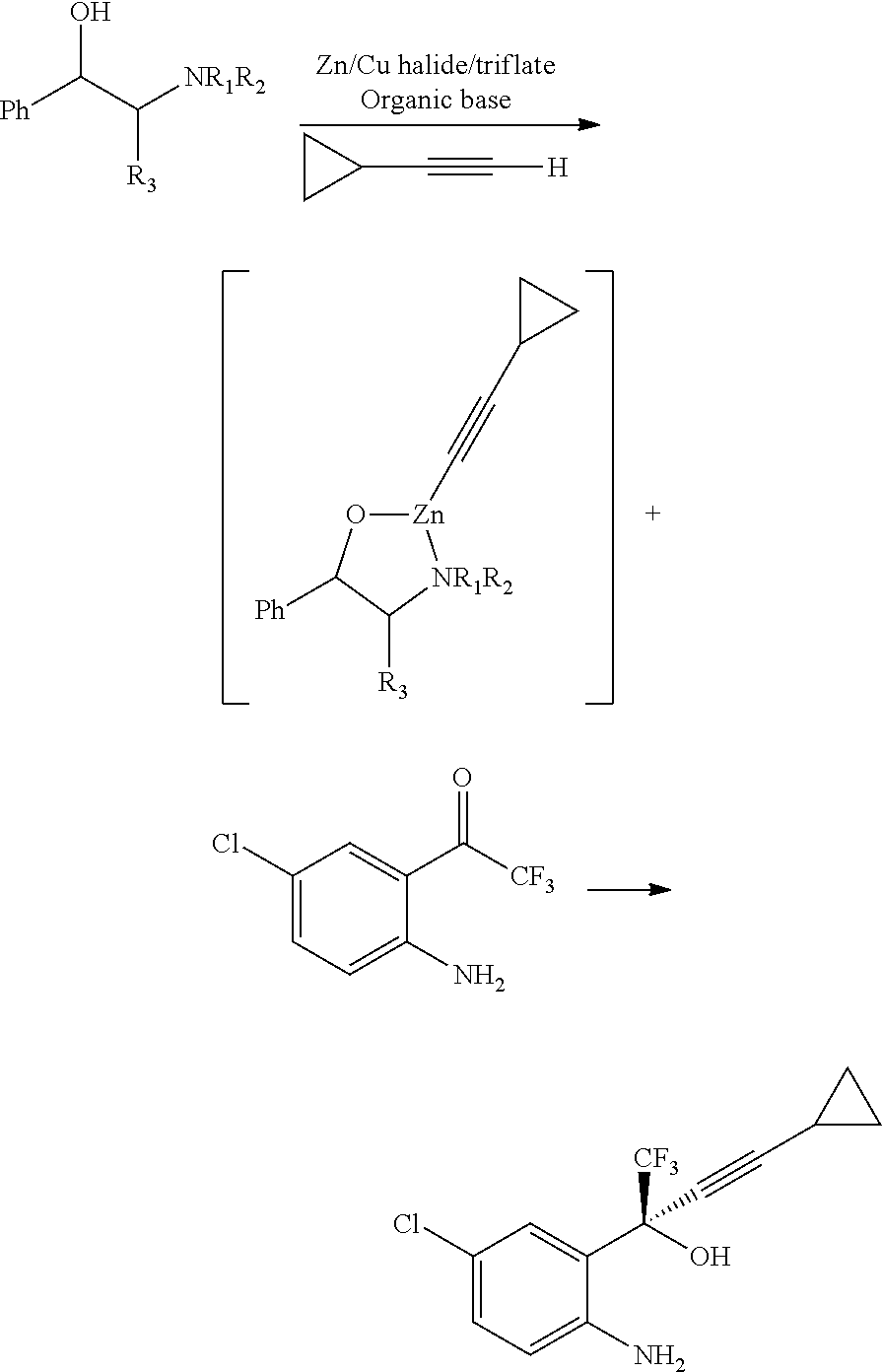
Reagents for asymmetric allylation, aldol, and tandem aldol and allyation reactions
A new class of reagents and method of use of the reagents in the reaction of the reagents with electrophilic compounds. The invention in one embodiment is directed to a method for the formation of an alcohol of the formula (I). The method includes reacting a reagent of the formula (II) with an aldehyde of the formula R10CHO to form the alcohol. X3 is one of O and C(R4)(R5). Each of X1 and X2 is independently O or N—R. Each of Ca and Cb is independently an achiral center, an (S) chiral center or an (R) chiral center. Ra and Rb are (i) each independently C1-10 alkyl, C6-10 aryl or C3-9 heteroaryl, or (ii) taken together to form a C3—C4 alkylene chain which together with Ca and Cb forms a 5-membered or 6-membered aliphatic ring. Rc and Rd are each independently hydrogen, C1-10 alkyl, C6-10 aryl or C3-9 heteroaryl. R is C1-10 alkyl, C6-10 aryl or C3-9 heteroaryl. Each of R1, R2, R3, R4, R5 is independently hydrogen, C1—C10 alkyl, C6-10 aryl, C3-9 heteroaryl, C1-10 alkoxy, C6-10 aryloxy, C1-10 dialkylamino, C1-10 alkyl-C6-10 arylamino, C1-10 diarylamino, or halogen. R6 is halogen, hydrogen, C1-10 alkyl, C6-10 aryl, C3-9 heteroaryl, C1-10 alkoxy, C6-10 aryloxy, C1-10 alkyl-C6-10 arylamino, C1-10 diarylamino, OSO2CF3 or SR. R10 may be C1-10 alkyl, C6-10 aryl, or C3-9 heteroaryl.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
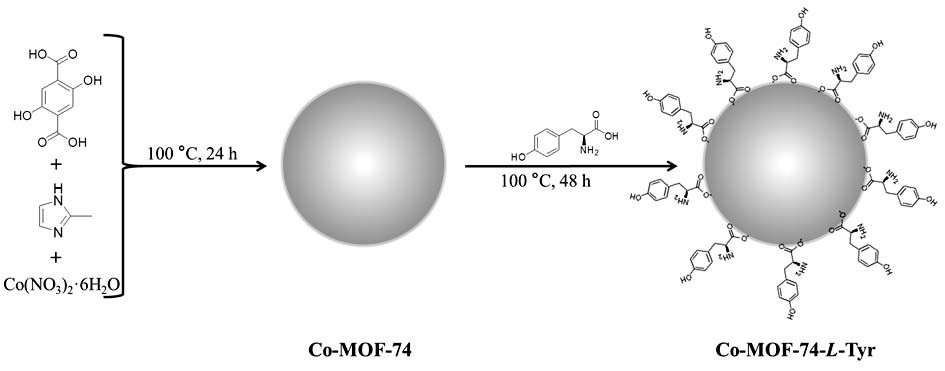
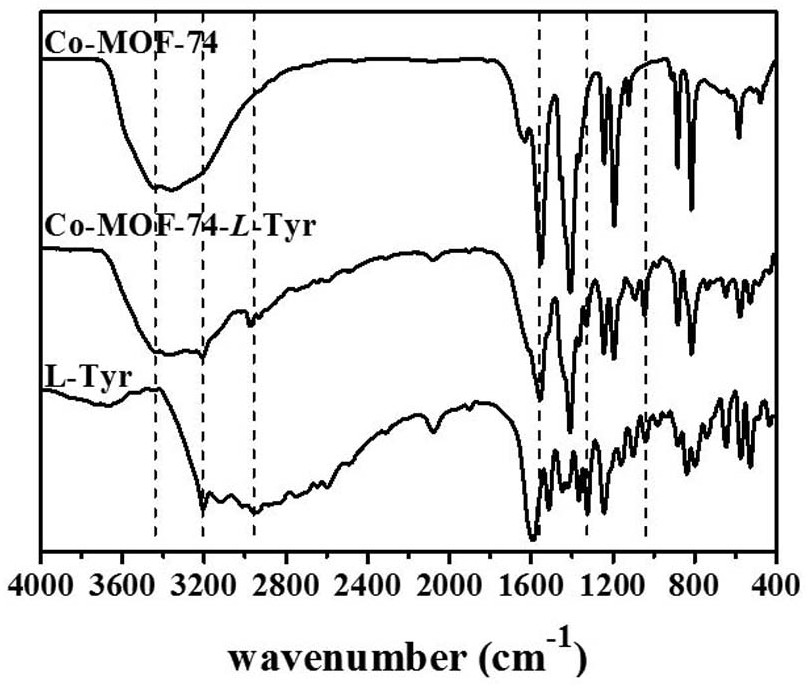
Chiral MOFs material and application of chiral MOFs material as chromatographic stationary phase in resolution of chiral drugs
ActiveCN113061264AGuaranteed separation effectUniform particlesIon-exchange process apparatusOther chemical processesAchiralityTyrosine
The invention discloses a chiral MOFs material and an application of the chiral MOFs material as a chromatographic stationary phase to resolution of chiral drugs; the synthesis of the chiral MOFs material comprises the steps: 1, preparing a 2-methylimidazole solution, a 2,5-dihydroxyterephthalic acid solution and a cobalt nitrate hexahydrate solution; 2, slowly dropwise adding a 2-methylimidazole solution and a 2,5-dihydroxy terephthalic acid solution into the cobalt nitrate hexahydrate solution in sequence, and after the reaction is finished, cooling, washing and drying to obtain Co-MOF-74; and 3, adding Co-MOF-74 into an L-tyrosine solution, carrying out a post-modification reaction, filtering, washing, activating, and drying to obtain the Co-MOF-74-L-Tyr. The achiral Co-MOF-74 is regulated and controlled by adding the regulator and changing the raw material molar ratio, the raw material adding sequence and the reaction conditions, it is ensured that the Co-MOF-74 is a microsphere which is regular in shape, uniform in particle, good in dispersity and uniform in size, the size and morphology of the Co-MOF-74 are close to those of filler SiO2 commonly used for a chromatographic column, the column pressure and the column efficiency of the chromatographic column can be effectively ensured, and the separation effect is ensured.
Owner:ZHENGZHOU UNIV
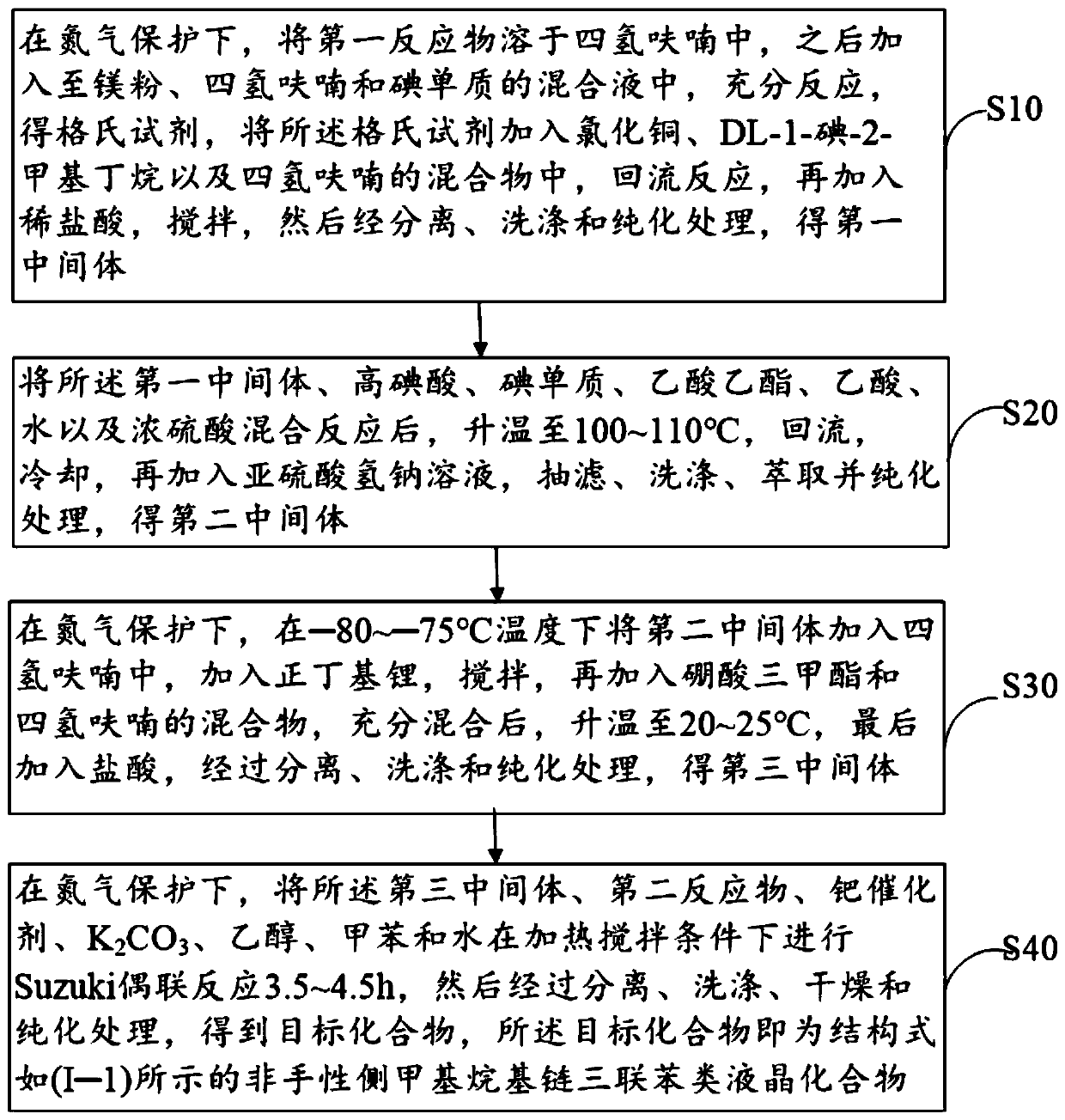
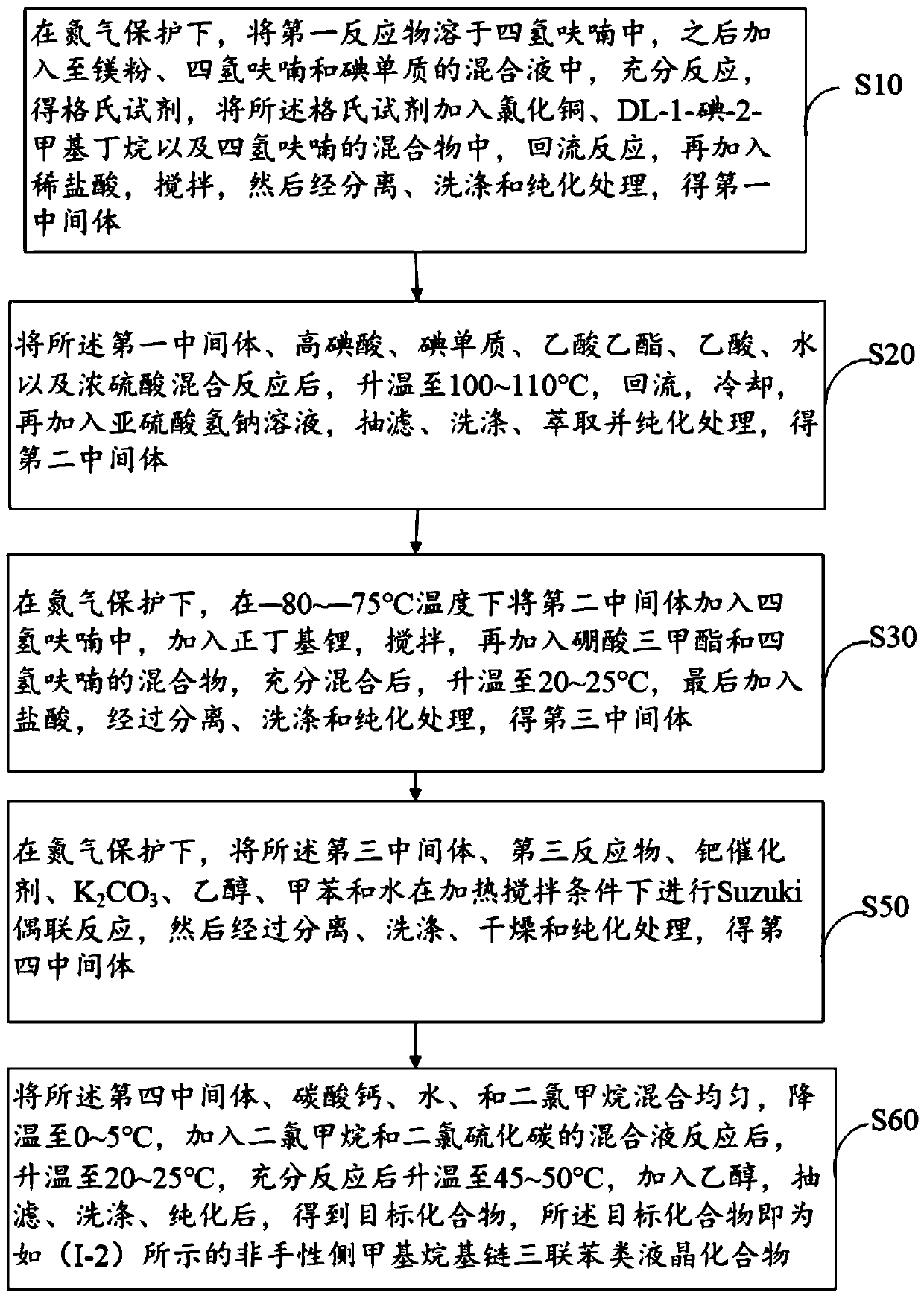
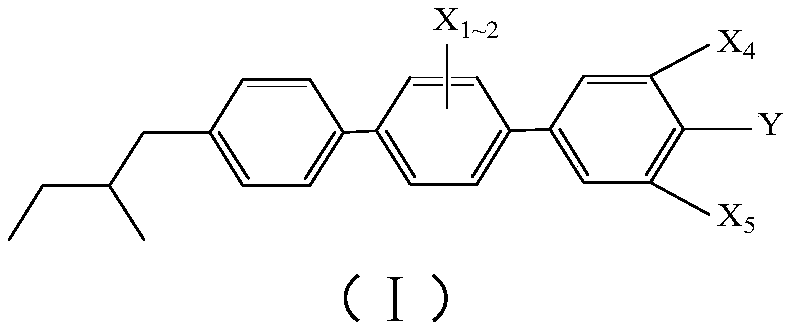
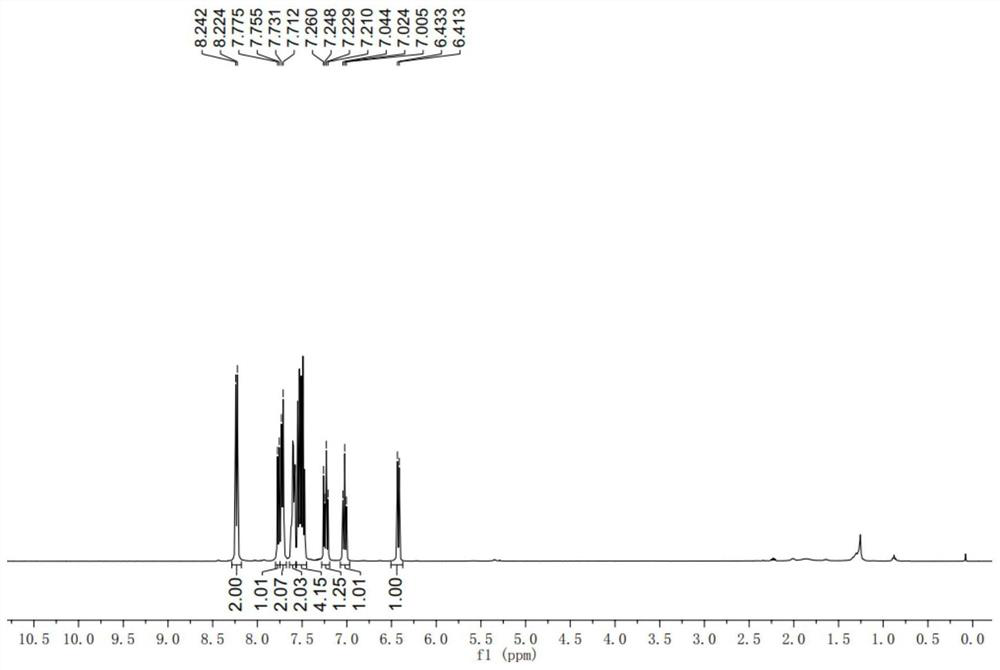
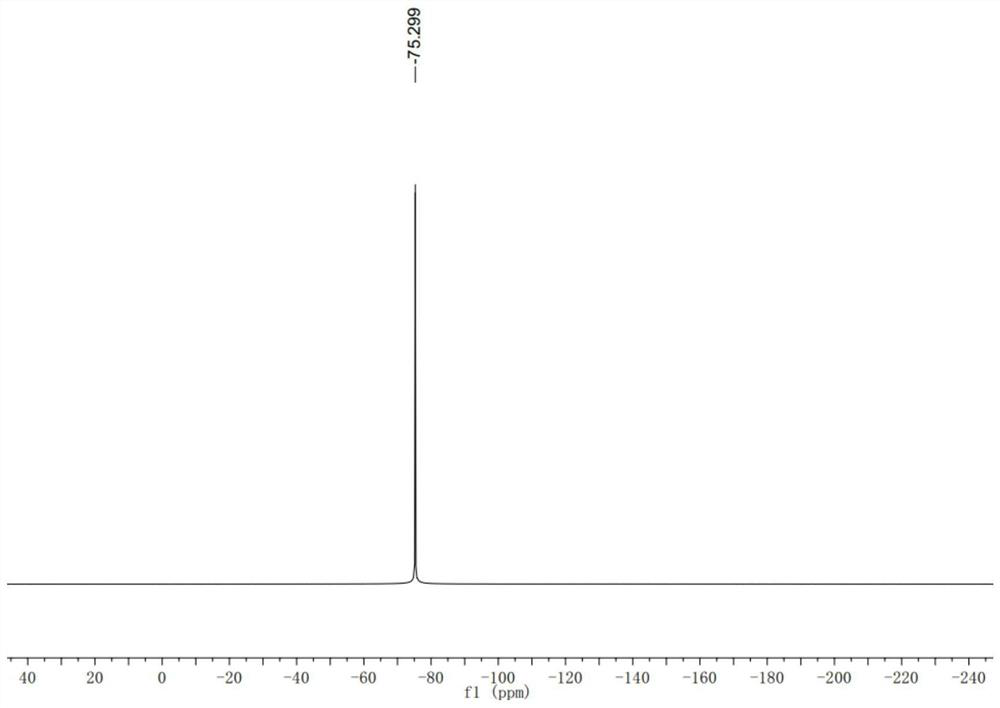
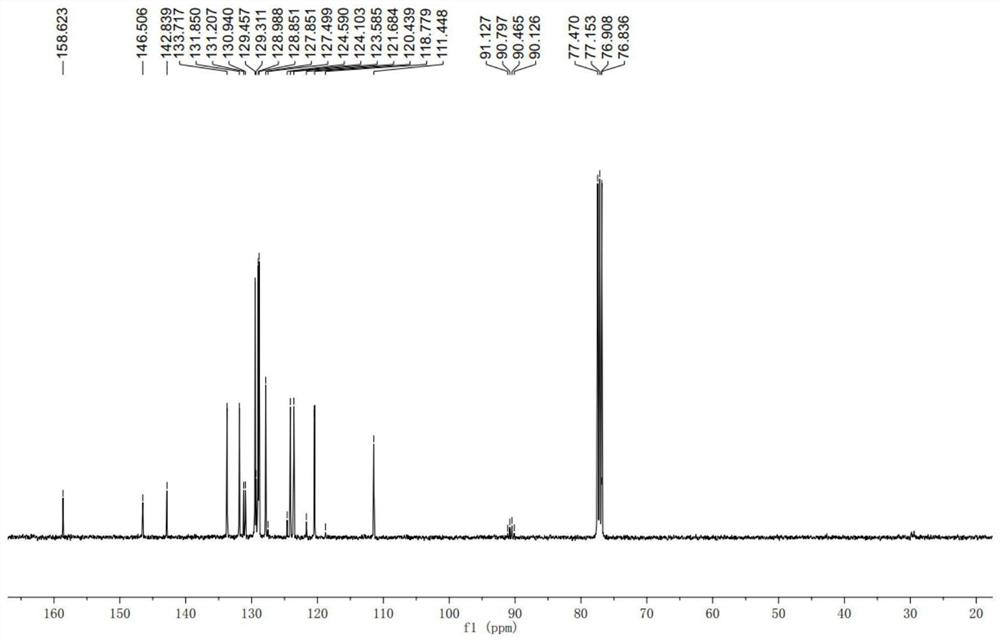
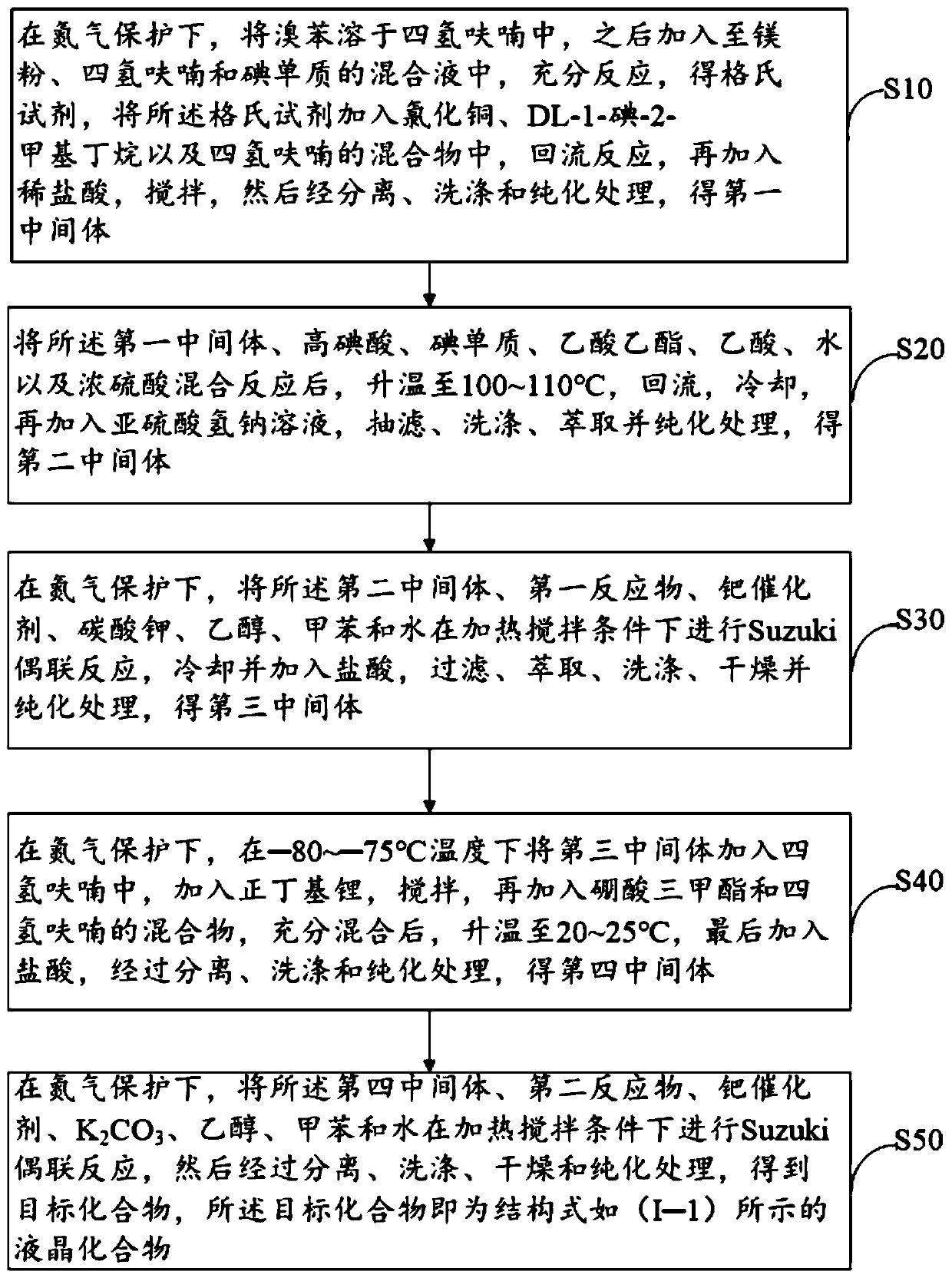
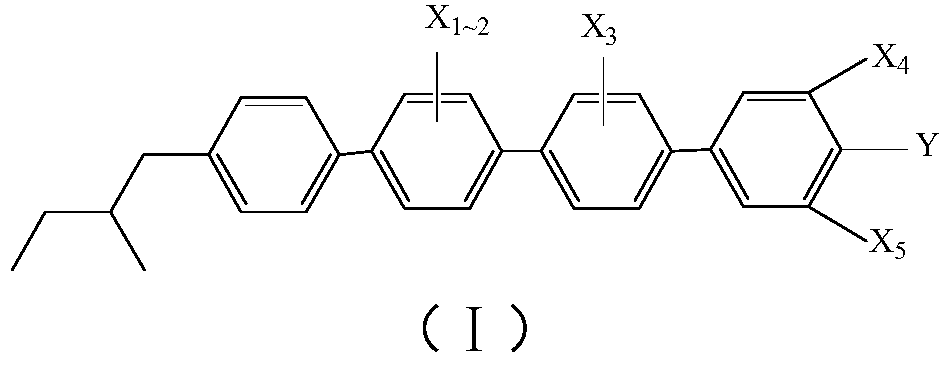
Achiral side methyl alkyl terphenyl liquid crystal compound, production method, liquid crystal composition and application
ActiveCN110746983AHigh dielectric constantHigh optical anisotropyLiquid crystal compositionsAmino preparation from aminesCrystallographyAchirality
The invention discloses an achiral side methyl alkyl terphenyl liquid crystal compound, a production method of the achiral side methyl alkyl terphenyl liquid crystal compound, a liquid crystal composition and application. The achiral side methyl alkyl terphenyl liquid crystal compound is of a molecular structure as shown in a formula (I) as shown in the specification. An achiral conformation branch end group of the compound (I) makes the compound (I) have a wide-temperature nematic phase state, and the branch end group improves the flexibility of a molecule, so that the compound (I) has a lowmelting point and large dielectric and optical anisotropy; due to a stable molecular structure of the compound (I), microwave absorption is little so that dielectric loss of a microwave device can bereduced; the compound (I) and other nematic liquid crystal compounds can be mixed to form a nematic liquid crystal material with large dielectric anisotropy and large optical anisotropy; and by applying the compound (I) into the microwave device, improvement of the phase modulation capability of the liquid crystal microwave device is facilitated, and a quality factor of the liquid crystal materialas a microwave medium is increased.
Owner:WUHAN POLYTECHNIC UNIVERSITY
N-heterocyclic carbene catalytic functionalized imine as novel 1, 4-dipole synthon and synthesis application thereof
ActiveCN112778328ALower synthesis costAchieving Stereodiversity SynthesisOrganic chemistryChemical synthesisAchirality
The invention relates to an N-heterocyclic carbene catalytic functionalized imine as a novel 1, 4-dipole synthon and application thereof, and belongs to the field of chemical synthesis. Under the mild reaction condition, a chiral N-heterocyclic carbene catalyst is used for catalyzing and activating aldimine, a novel aza 1, 4-dipole synthon is obtained under the oxidation condition, and the novel organic synthon can be further subjected to 4 + 2 cyclization reaction with trifluoroacetophenone, isatin and an isatin-derived imine substrate to generate a heterocyclic compound with a novel structure and a chiral quaternary carbon center. According to the method disclosed by the invention, the N-heterocyclic carbene catalyst with the same chiral configuration and an achiral thiourea catalyst are used for co-catalysis, so that three-dimensional diverse synthesis of the trifluoroacetophenone compound can be realized. The method is mild in condition and efficient in reaction and has good substrate universality, and the reported aldimine-derived 1, 4-aza dipole synthon provides an important method for synthesizing various functional nitrogen heterocyclic compounds and has the potential of being applied to industrial production.
Owner:NANJING UNIV OF TECH
Method for producing 1-amino-1-alkoxycarbonyl-2-vinylcyclopropane
ActiveUS9061991B2Carbamic acid derivatives preparationOrganic compound preparationLeaving groupCyclopropane
It is an object of the present invention to provide a novel method for producing (1R,2S) / (1S,2R)-1-amino-1-alkoxycarbonyl-2-vinylcyclopropane which is useful as a synthetic intermediate of therapeutic agents for hepatitis C and a synthetic intermediate thereof. According to the present invention, when a trans-2-butene derivative having a leaving group at each of the 1- and 4-positions is reacted with a malonic ester in the presence of a base, a specific amount of an alkali metal alkoxide or an alkali metal hydride is used as the base, and further a specific amount of a malonic ester is used to produce a cyclopropane diester, and further, chiral or achiral 1-amino-1-alkoxy-carbonyl-2-vinylcyclopropane and a salt thereof are synthesized using the cyclopropane diester.
Owner:API CORP (JP)
Achiral polymer-based organic electroluminescent circularly polarized light-emitting device
PendingCN113178539ASolid-state devicesSemiconductor/solid-state device manufacturingAchiralityThiadiazoles
The invention provides an achiral polymer-based organic electroluminescent circularly polarized light-emitting device which comprises an anode, a hole injection layer, a light-emitting layer, an electron transport layer, an electron injection layer and a cathode which are sequentially superposed. The material of the light-emitting layer is poly (9, 9-dihexylfluorene-co-benzothiadiazole) induced by circularly polarized light. The invention provides an OLED which is based on an achiral polymer (F6BT) and can directly and preferentially emit left-handed or right-handed circularly polarized light. The spiral response of the OLED is easy to control, and the spiral response is not only on the rotational property of inducing the circularly polarized light, but also on the wavelength of inducing the circularly polarized light. According to the OLED provided by the invention, a simpler, more convenient and more efficient design method is provided for realizing the circular polarization luminescence of a traditional achiral compound, and a foundation is laid for preparing a large-area and high-quality chiral micro-nano photoelectric device.
Owner:UNIV OF SCI & TECH OF CHINA
Preparation method and application method of chiral-atom-free helically chiral all-cis polyphenylacetylene derivative materials
ActiveCN108383934AGood chiral resolution performanceLow costIon-exchange process apparatusSilicon organic compoundsSolventChiral stationary phase
The invention provides a preparation method and an application method of chiral-atom-free helically chiral all-cis polyphenylacetylene derivative materials. The preparation method comprises: dissolving a non-optically active poly 3,5-dihydroxymethyl-4-substituted phenylacetylene derivative in a chiral solvent to prepare a solution, replacing the chiral solvent in the solution with an achiral solvent through a membrane permeation method, and carrying out precipitation washing to prepare a series of the chiral-atom-free all-cis helical poly 3,5-dihydroxymethyl-4-substituted phenylacetylene derivative materials with helical chirality and the corresponding coating type high-performance liquid chromatography chiral stationary phase. According to the present invention, the used chiral reagent ofthe preparation method can be repeatedly used, the preparation method has characteristics of low cost and simple operation, the chiral stationary phase has good chiral splitting performance, and themethod has broad application prospects.
Owner:HARBIN ENG UNIV
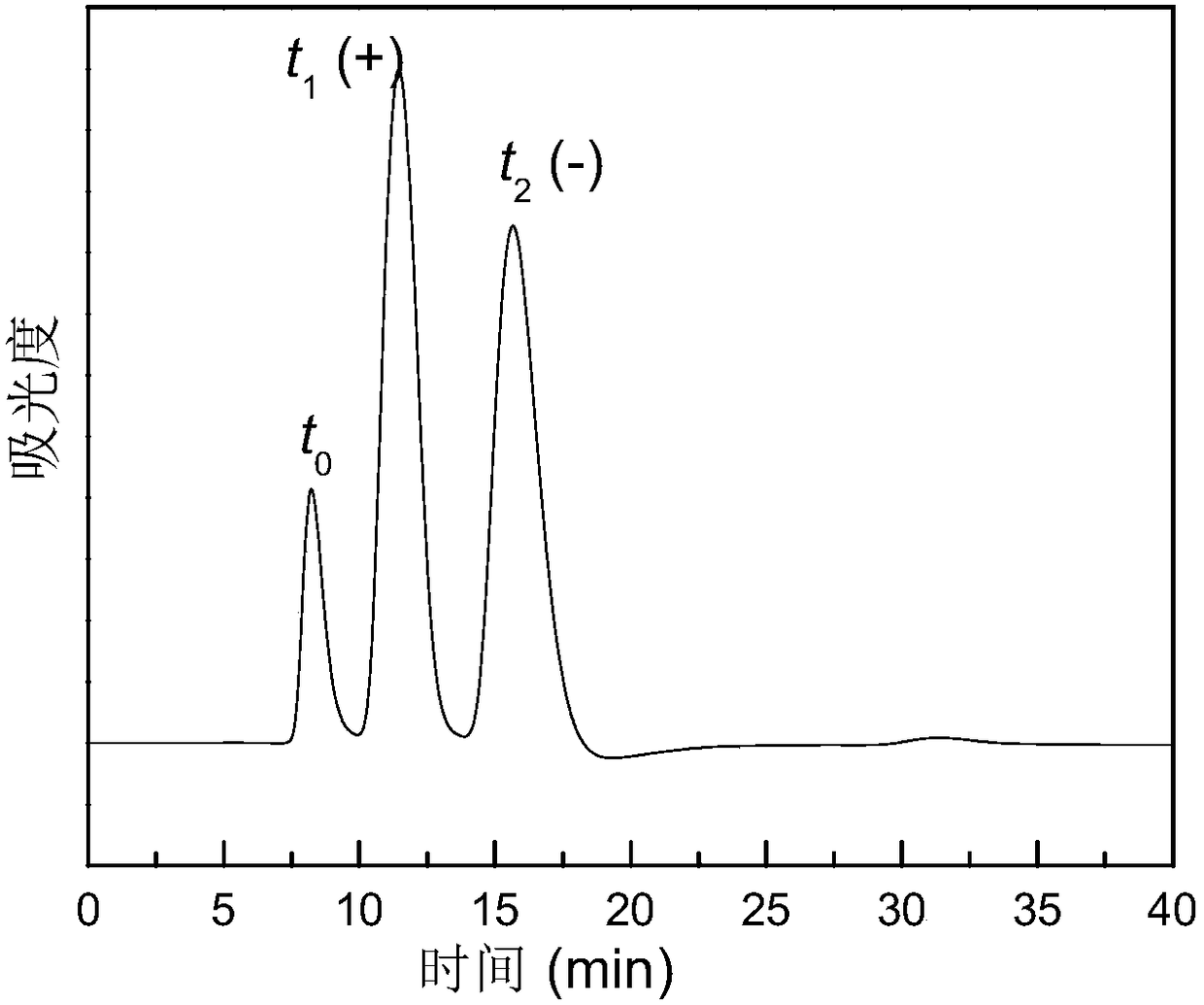
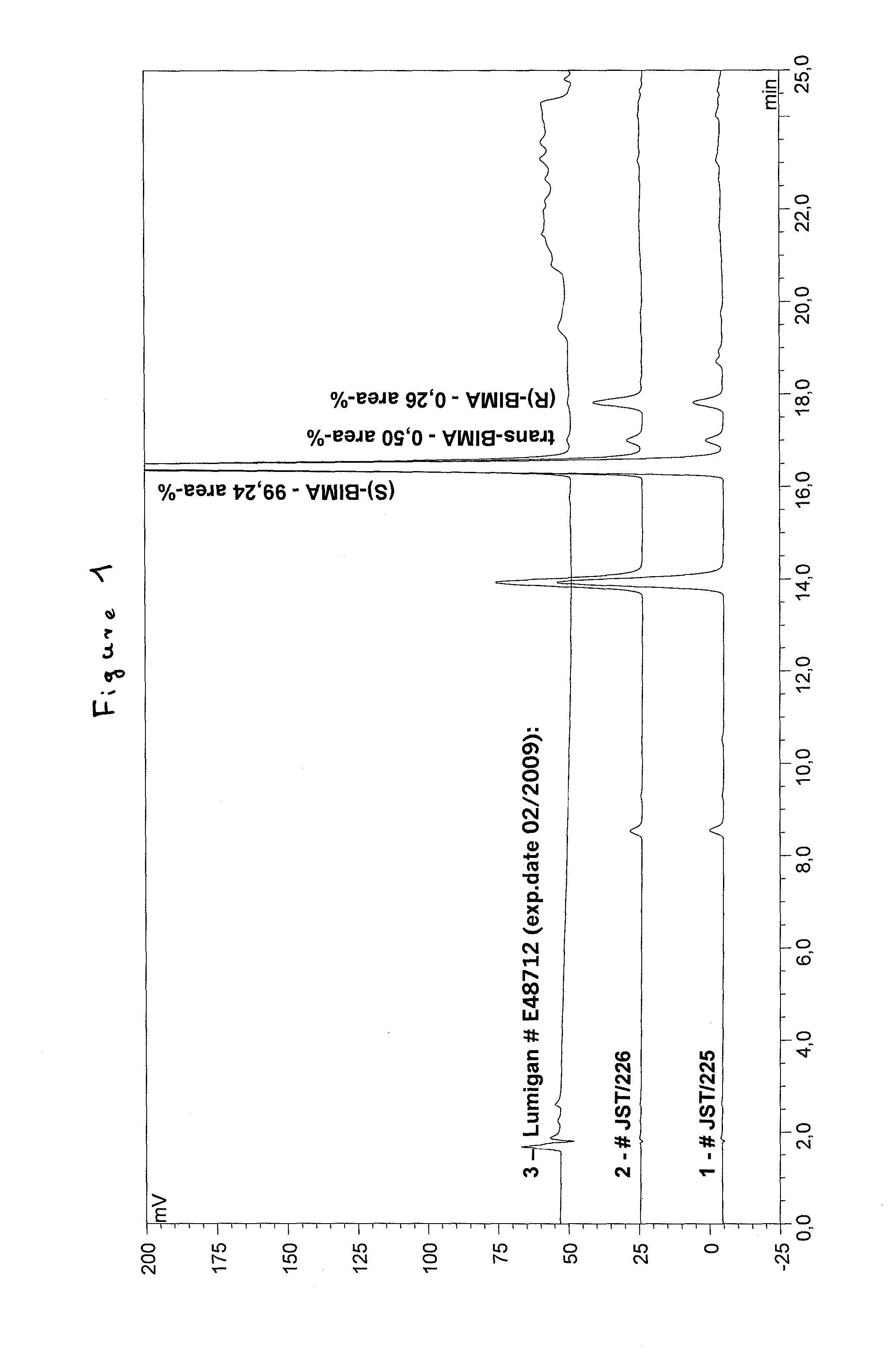
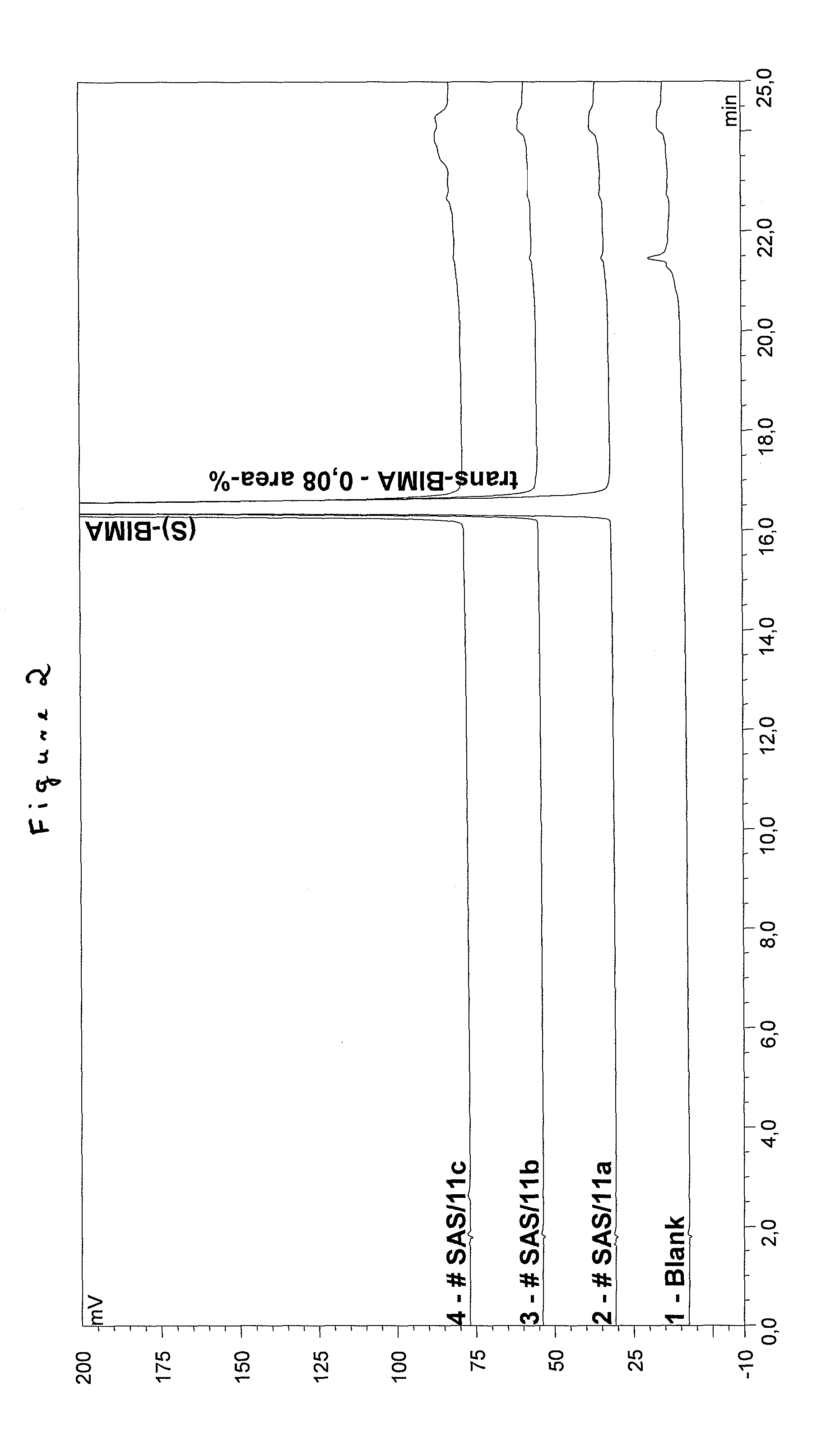
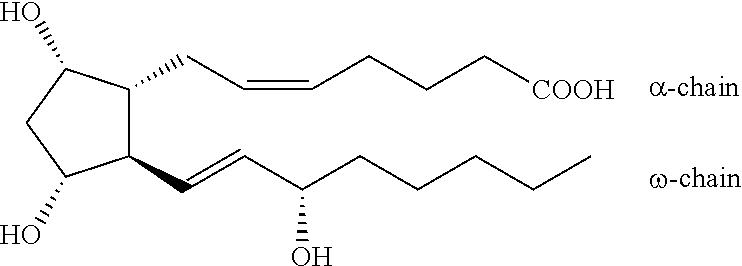
Process for the production of bimatoprost
InactiveUS8772544B2High content of impuritiesKeep for a long timeOrganic compound preparationCarboxylic acid amide separation/purificationAlcoholSolvent
The present invention relates to a process for the purification of crude bimatoprost to obtain pure bimatoprost comprising a chromatography, preferably a chromatography using an achiral stationary phase and an eluent comprising an alcohol and an apolar solvent; and crystallization of the product obtained the chromatography to obtain pure bimatoprost.
Owner:SANDOZ AG
Chiral azobenzene polymer film as well as preparation method and application thereof
The invention discloses a chiral azobenzene polymer film as well as a preparation method and application thereof. A side chain-type azobenzene polymer is prepared into a film; and chiral reagent induction is carried out to obtain a chiral azobenzene polymer film. The method comprises the following steps: firstly, synthesizing an azobenzene random copolymer with hydroxyl at the tail end of a side chain through a series of organic synthesis reactions and RAFT polymerization, and carrying out detailed investigation on the molecular weight and the liquid crystal performance of the polymer by utilizing characterization means such as nuclear magnetism, GPC, DSC, POM and XRD; and preparing a polymer film in a spin-coating manner, and performing chiral induction on the polymer film by selecting chiral limonene steam to obtain an optically active polymer film. The chirality is transmitted from a chiral reagent to an achiral substance. The chiral solvent induction method provided by the invention is simple to operate, and avoids the complex synthesis steps of the chiral polymer and the use of expensive chiral reagents.
Owner:SUZHOU UNIV
Preparation method of chiral optical thin film with porphyrin intercalation DNA and hydrotalcite compound
InactiveCN104449676AOrderly accumulationGood for signal amplificationLuminescent compositionsHigh densityPorphyrin
The invention discloses a method for preparing a chiral optical thin film with a porphyrin intercalation DNA and a hydrotalcite compound. The technical scheme of the invention is that the method comprises the following steps: firstly, stripping hydrotalcite nano-particles into nano-sheets serving as construction units; secondly, assembling the hydrotalcite nano-sheets with positive charges and a DNA with negative charges by using a layer-by-layer assembly technology; and finally, inserting achiral optical molecular porphyrin into a spiral cavity of the DNA to obtain a thin film material inducing chiral luminescence. A hydrotalcite matrix provides a closed and stable microenvironment for DNA molecules. Ordered packing of high-orientation and high-density DNA arrays can be realized, which is favorable for signal amplification of optical molecules. The achiral optical molecular porphyrin obtains a chiral luminescence characteristic after entering the chiral DNA spiral cavity. Therefore, the invention provides a new method for preparing a chiral optical thin film material.
Owner:BEIJING UNIV OF CHEM TECH
Stretchable blue-phase liquid crystal elastomer and preparation method thereof
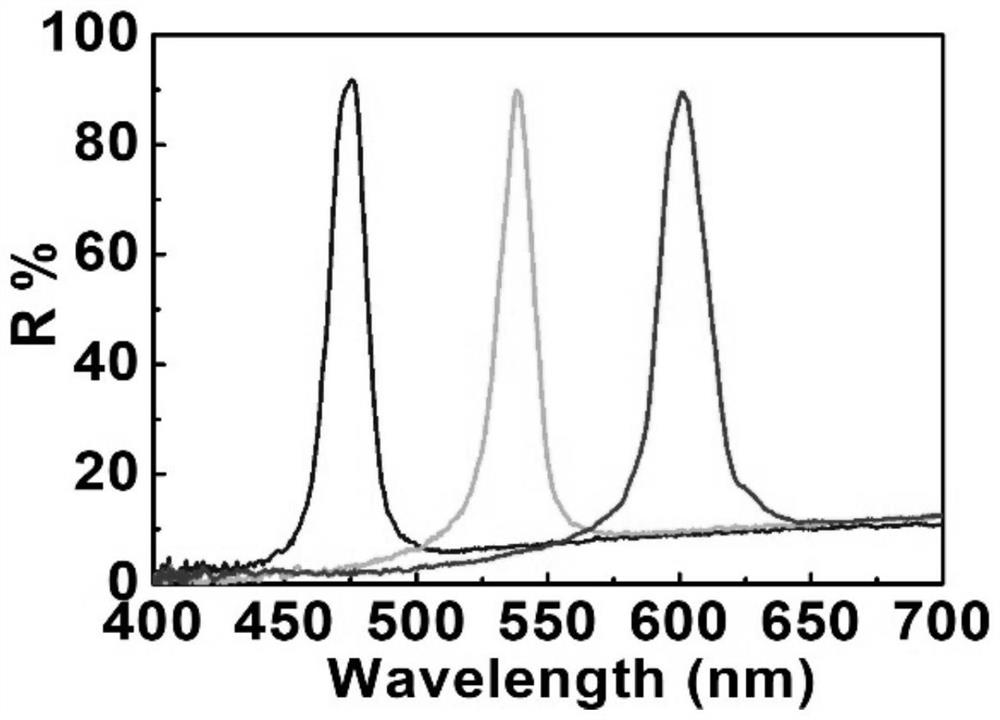
PendingCN114164008AEven elastic coloringImprove reflectivityLiquid crystal compositionsPolymer scienceAchirality
The invention provides a stretchable blue-phase liquid crystal elastomer and a preparation method thereof. The stretchable blue-phase liquid crystal elastomer is prepared from the following raw materials: achiral micromolecular liquid crystal, a photopolymerizable monomer, a chiral material, a photoinitiator and a click reaction crosslinking monomer. In the preparation method, liquid crystal molecules can be induced to be self-assembled to construct a blue-phase liquid crystal structure in a click chemical reaction process. The blue-phase liquid crystal elastomer has a synergistic response to deformation and color change and has good mechanical ductility, and the mechanically reversible reflection color of the elastomer can be flexibly adjusted in almost the whole visible light wave band and can be stretched to two times or more of the original length.
Owner:HEBEI UNIV OF TECH
Method for asymmetric functionalization of nickel-hydrogen catalytic olefin migration promoted by ligand relay strategy
ActiveCN112939750APromotes isomerizationFacilitates asymmetric couplingGroup 4/14 element organic compoundsOrganic compound preparationNickel saltAchirality
The invention discloses a method for asymmetric functionalization of nickel-hydrogen catalytic olefin migration promoted by a ligand relay strategy. Under the action of a metal nickel salt, an achiral ligand, a chiral ligand, alkali, a hydrogen source, an additive and the like, olefin and various electrophilic reagents are dissolved in an organic solvent for reaction, and the chiral compound with excellent regioselectivity and enantioselectivity is obtained. According to the method, the chiral compound containing various functional groups can be efficiently synthesized, the product has high enantioselectivity, the raw materials are simple and easy to obtain, and operation is easy and convenient.
Owner:NANJING UNIV
Chiral menthyl liquid crystal oligomer film and preparation method thereof
ActiveCN113265078AImprove thermal stabilityWide LCD rangeLiquid crystal compositionsCrystallographyLiquid crystalline
The invention discloses a chiral menthyl liquid crystal oligomer film and a preparation method thereof, the chiral menthyl liquid crystal oligomer is prepared by copolymerizing chiral menthyl liquid crystal monomers, achiral rod-like monomers and polymethyl hydrogen-containing linear siloxane in different molar ratios, the structural formula of the chiral menthyl liquid crystal oligomer is as shown in formula III, and the chiral menthyl liquid crystal oligomer film with bright color at room temperature is obtained by high-temperature quenching. The chiral menthyl liquid crystal oligomer film has high thermal stability and a wide liquid crystal interval, can retain the color of cholesteric liquid crystal at room temperature, presents bright color, and has wide application.
Owner:XUZHOU UNIV OF TECH
Optically isotropic liquid crystal composition and optical switching device using same
InactiveUS20200299581A1High transparencyHigh isotropyLiquid crystal compositionsWave based measurement systemsAchiralityDielectric permittivity
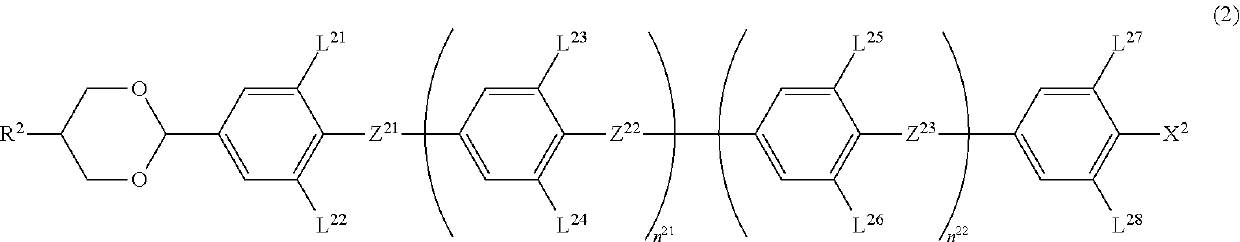
A device using a liquid crystal medium exhibiting an optically isotropic liquid crystal phase, particularly, a blue phase liquid crystal medium for polarized light control application, in which reduction of an effective dielectric constant in a high frequency region is suppressed. A liquid crystal composition contains achiral component T, and has a liquid crystal phase optically exhibiting isotropy, in which achiral component T contains at least one compound selected from the group of compounds represented by formula (1) as a first component, at least one compound selected from the group of compounds represented by formula (2) and formula (3) as a second component, and is used for optical switching for controlling retardation by electric field-induced birefringence.
Owner:JNC CORP +1
Liquid crystal medium, optical device and liquid crystal compound
InactiveUS20160304784A1High clearing pointWide Nematic Temperature RangeLiquid crystal compositionsOrganic chemistryCrystallographyDielectric anisotropy
A liquid crystal medium that has stability to heat, light and so forth, a wide temperature range of a liquid crystal phase and significantly large dielectric anisotropy to develop an optically isotropic liquid crystal phase is required. Moreover, various optical devices that can be used in a wide temperature range, and have a short response time, a large contrast ratio and a low drive voltage are required.A liquid crystal composition contains an achiral component T containing at least one compound (1) represented by formula (1) and a chiral agent to develop an optically isotropic liquid crystal phase:(wherein, in formula (1), R1 is alkyl having 1 to 12 carbons, L1, L2 and L3 are each independently hydrogen, fluorine or chlorine; and Y1 is fluorine, for example).
Owner:JNC CORP +1
Chiral azobenzene polymer cross-linked film as well as preparation method and application thereof
The invention discloses a chiral azobenzene polymer cross-linked film as well as a preparation method and application thereof. The chiral azobenzene polymer cross-linked film is a novel super-molecular chiral construction and chiral immobilization method based on an achiral side chain type azobenzene polymer film. According to the invention, limonene steam is utilized to carry out chiral inductionon a polymer film at a high temperature, and then formaldehyde steam is utilized to carry out acetalation reaction with hydroxyl in an acidic environment to realize cross-linking; and then the difference of the stability of the super-molecular chirality of the polymer film before and after crosslinking is studied under the conditions of light, heat and good solvent dissolution, and the self-repairing performance of the microcosmic spiral chirality is researched. The cross-linked film prepared by the invention has good chiral performance and excellent solvent resistance, heat resistance and self-repairing performance.
Owner:SUZHOU UNIV
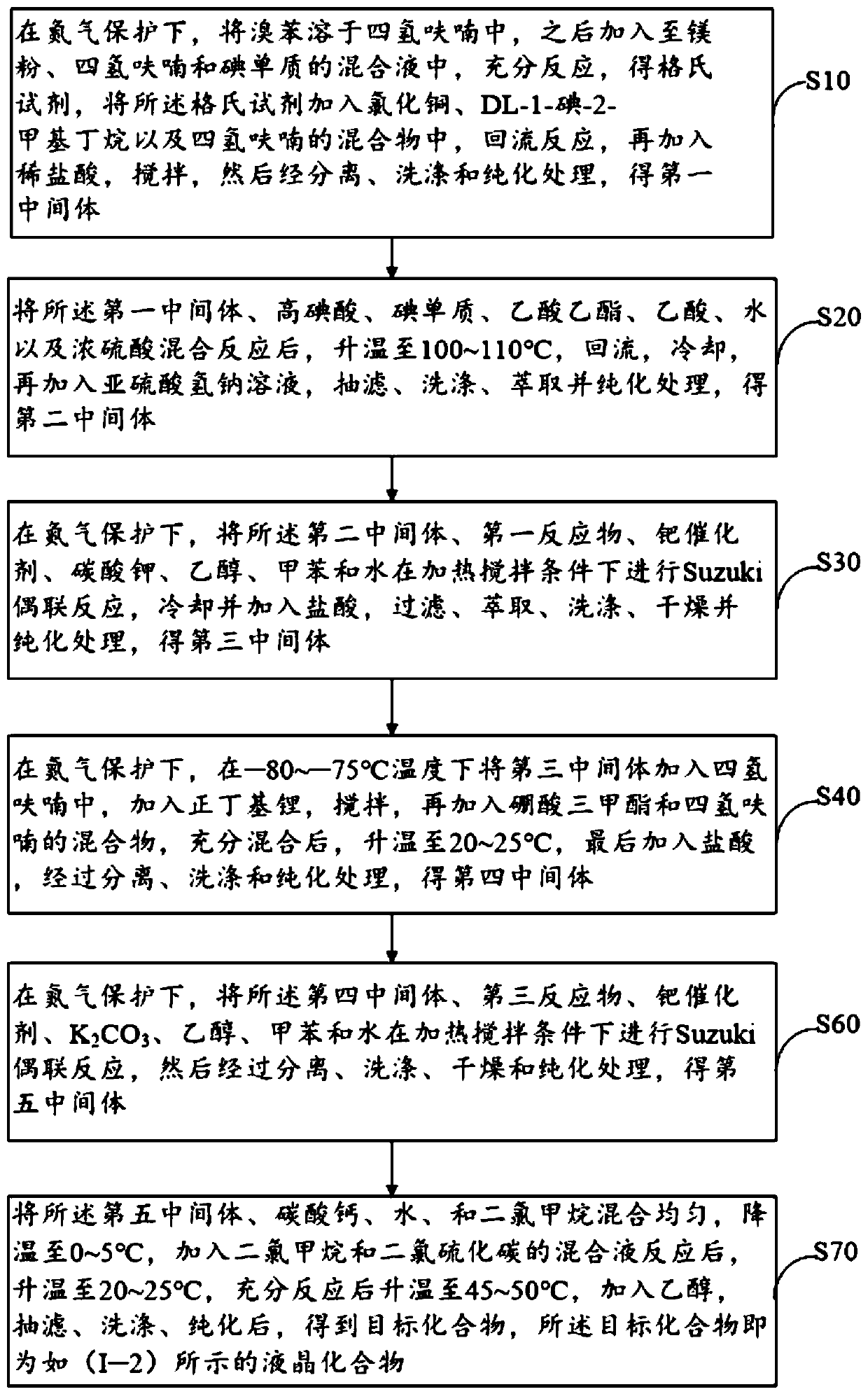
Achiral side methylalkyl quaterphenyl liquid crystal compound and preparation method, liquid crystal composition and microwave communication device
ActiveCN110746982ALow melting pointHigh melting pointLiquid crystal compositionsAmino preparation from aminesCrystallographyAchirality
The invention discloses an achiral side methylalkyl quaterphenyl liquid crystal compound and a preparation method thereof, a liquid crystal composition and a microwave communication device. The achiral side methylalkyl quaterphenyl liquid crystal compound has a structure shown by a structural formula (I). The compound (I) has quaterphenyl and branched chain methylalkyl structural units, because the branched chain methylalkyl group has an achiral conformation, the molecular flexibility of the quaterphenyl liquid crystal compound is increased, and thereby the achiral side methylalkyl quaterphenyl liquid crystal compound (I) has a stable structure, and has the advantages of relatively low melting point, large optical anisotropy, wide temperature nematic phase, and the like. The quaterphenyl liquid crystal compound is applied in high dielectric anisotropic liquid crystal materials, the reduction of the dielectric loss of a liquid crystal microwave device is facilitated, the microwave phasemodulation capability is increased, and the quality factor of the liquid crystal materials is improved.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Self-supporting blue-phase liquid crystal film and preparation method thereof
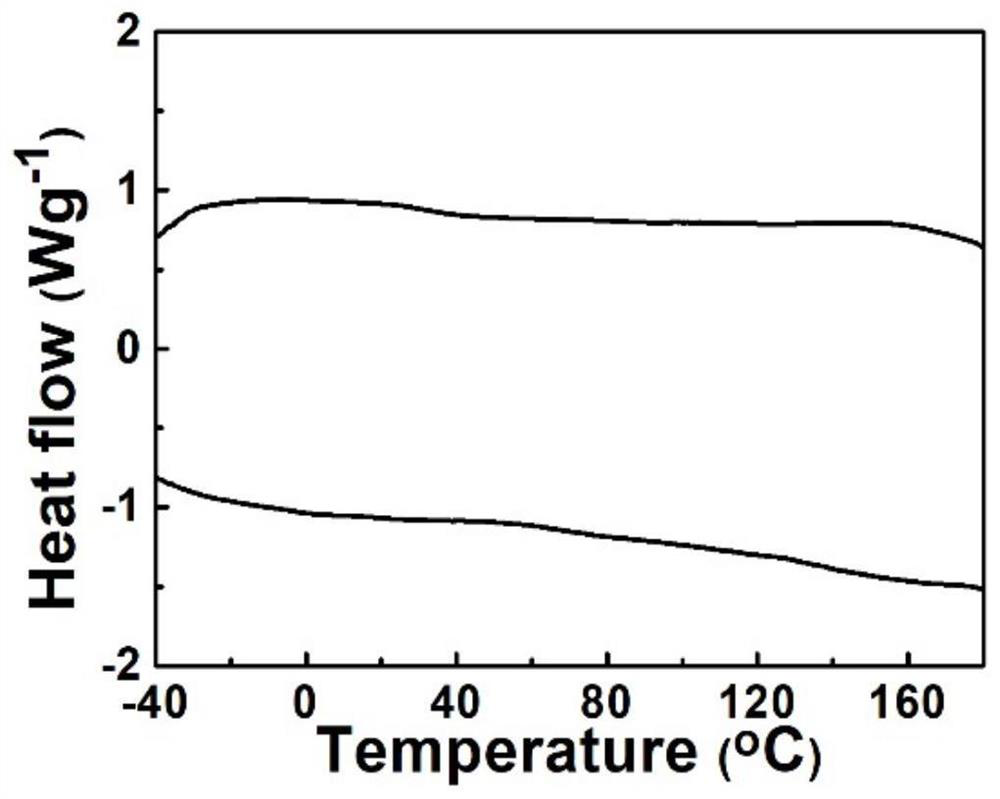
ActiveCN111665653ABroaden the temperature range of the blue phaseStable blue phase structureNon-linear opticsCrystallographyLiquid crystalline
The invention provides a self-supporting blue-phase liquid crystal film. The self-supporting blue-phase liquid crystal film comprises the following components in percentage by mass: 3-7% of chiral liquid crystal, 24-93% of achiral polymerizable liquid crystal, 0-65% of achiral small molecular liquid crystal and 4-12% of non-liquid crystal cross-linking agent. The blue-phase liquid crystal film canrealize self-supporting and is not limited by a liquid crystal box, so that the application range of the blue-phase liquid crystal film is greatly expanded; the temperature range of the blue phase isnot lower than 220 DEG C, and the reflection band gap and the reflectivity can be regulated and controlled by adjusting the proportion of all the raw materials. In addition, the blue-phase liquid crystal film is simple to prepare and operate, low in cost and suitable for large-scale preparation.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
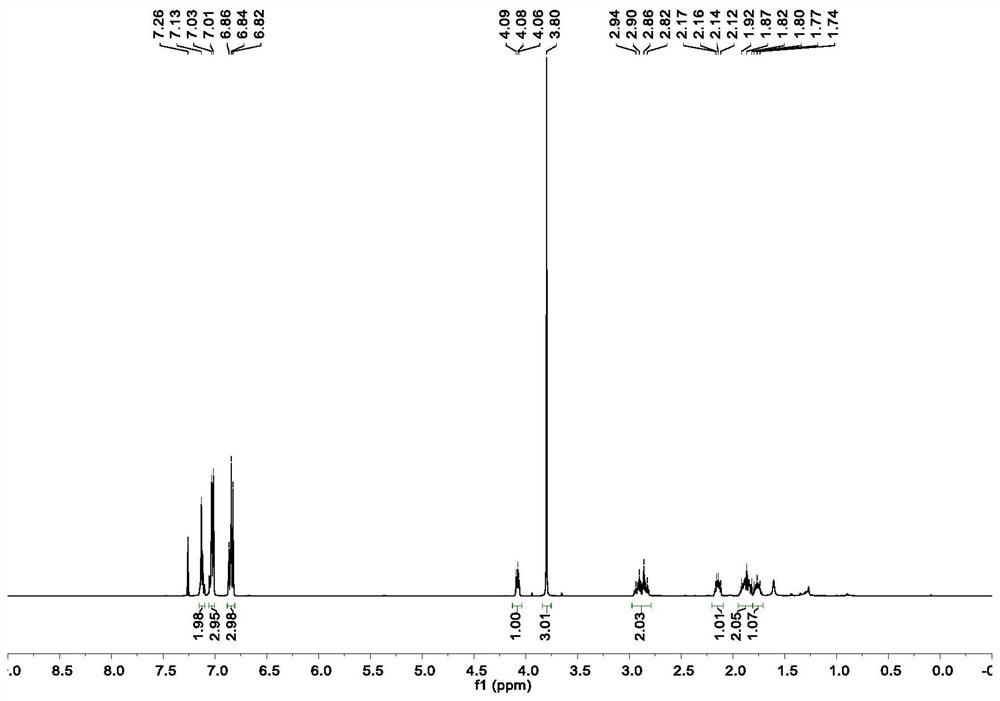
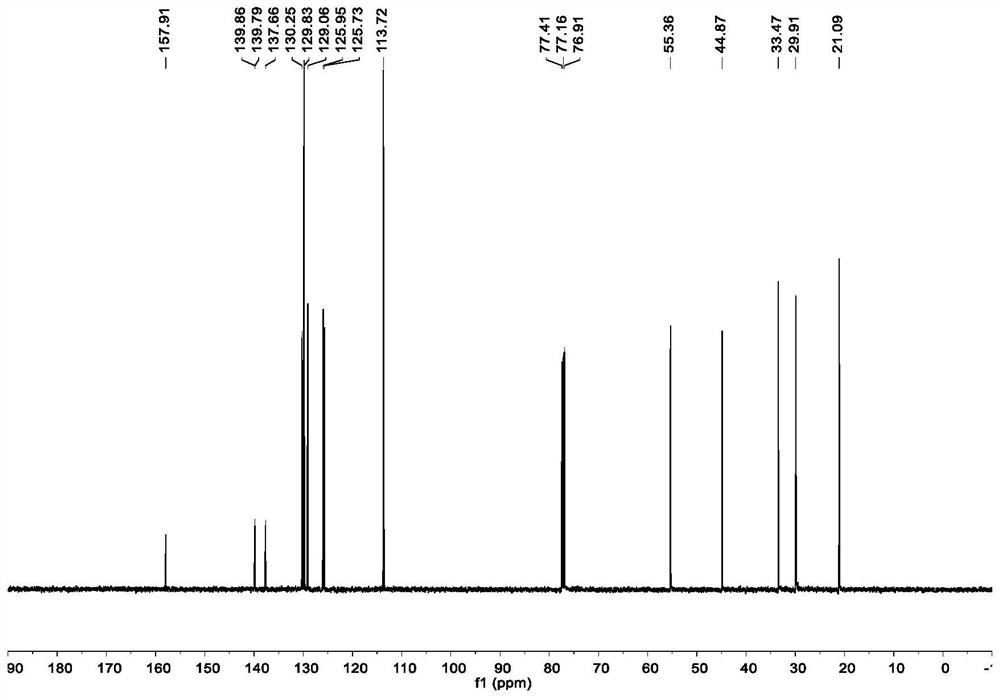
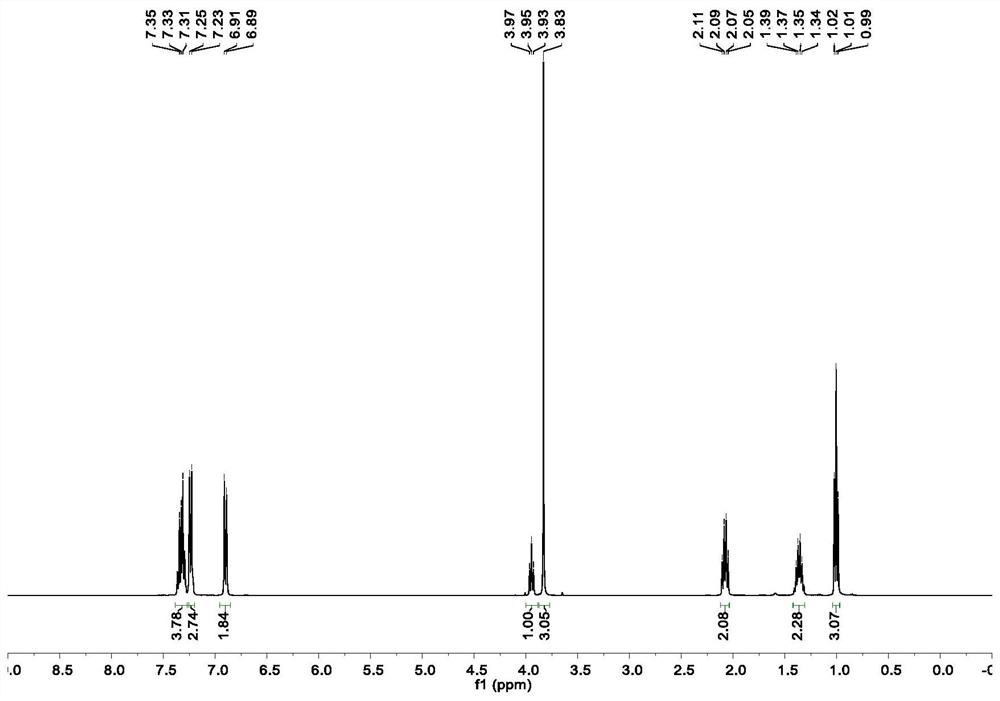
Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane
The invention provides a method for preparing a moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane, and relates to the technical field of organic synthesis. According to the method, the azaphthalide is used as a raw material, and due to the ring structure of the azaphthalide, the other two non-corresponding chiral isomers which are not needed in chiral reduction are almost not generated; the chiral purity of the intermediate obtained through reduction is very high, resolution is not needed, and (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane can be obtained directly through ammonolysis, reduction, chlorination and cyclization subsequently. According to the method provided by the invention, chiral resolution is not needed, the process is simple, the process steps are short, the cost is low, and the product is high in chiral purity and high in total yield. Furthermore, in the subsequent product salifying and refining step, carboxylic acid with a chiral structure does not need to be used for salifying, and common achiral carboxylic acid can be used for refining, so the product purity is further improved.
Owner:TAIZHOU BIOMEDICAL & CHEM IND RES INST CO LTD
Single chiral center catalyst, preparation method thereof and method for catalytically synthesizing chiral alcohol compounds and chiral alpha-allyl alcohol
ActiveCN112675920AHigh catalytic activityImprove stabilityGroup 8/9/10/18 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsAchiralityPtru catalyst
The invention relates to a single chiral center catalyst, a preparation method thereof and a method for catalytically synthesizing chiral alcohol compounds and chiral alpha-allyl alcohol. The single chiral center catalyst has a structure as shown in the following general formula shown in the description, wherein in the formula, Z is -S-, -N = CH- or -CH = CH-. According to the catalyst, the achiral diphosphorus ligand is adopted, and chiral elements of the diamine ligand are reserved and adjusted at the same time, so the chirality of catalyzing asymmetric reaction can be independently controlled, and the synthesis difficulty and cost of the chiral catalyst are greatly reduced; the catalyst can be used for catalyzing asymmetric transfer hydrogenation of aryl-aryl substituted ketone compounds, heterocyclic aromatic-aryl substituted ketone compounds and heterocyclic aromatic-heterocyclic aryl substituted ketone compounds, preparing chiral secondary alcohol compounds and achieving kinetic resolution of racemic alpha-allyl alcohol compounds.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
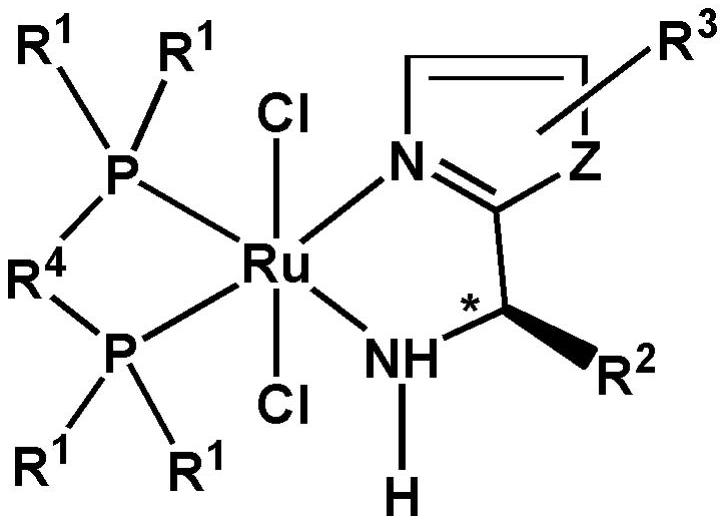
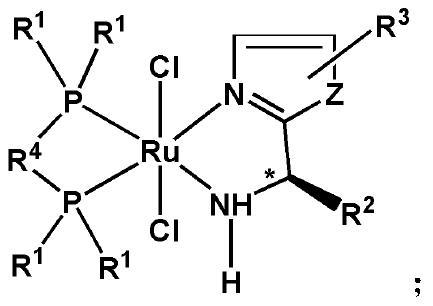
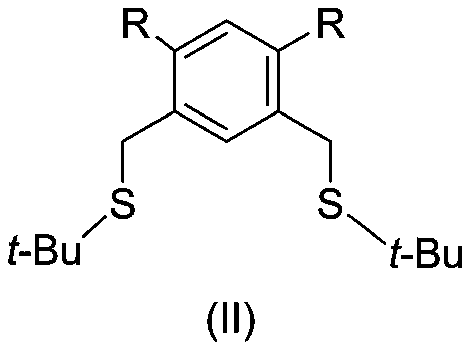
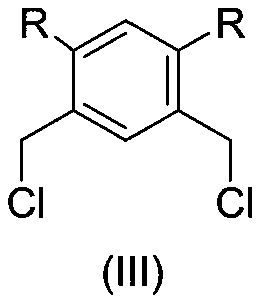
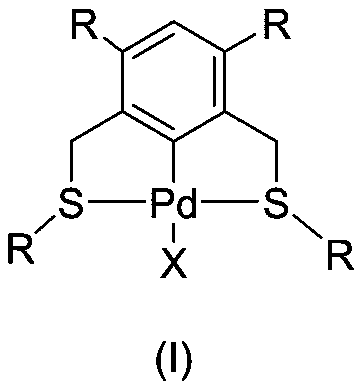
Achiral SCS type pincer-like compound and palladium complex thereof
InactiveCN111039835AHigh yieldHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsPalladium organic compoundsAchiralityPtru catalyst
The invention discloses an achiral SCS type pincer-like compound and a palladium complex thereof. Chemical formulas of the achiral SCS type pincer-like compound (II) and the palladium complex (I) of the achiral SCS type pincer-like compound (II) are respectively shown in the following general formulas (II) and (I). The palladium complex (I) (wherein X represents -Cl, and R and R1 represent a hydrogen atom or an alkyl group having 1-4 carbon atoms) is synthesized by a coordination reaction between the compound (II) and a divalent palladium salt. The palladium complex (I) (wherein X is a group selected from -Br, -I,- OAc, -OTf, -BF4 and -SbF6) is synthesized by an anion exchange reaction of the palladium complex (I) in which X represents -Cl. The palladium complex (I) can be used as a catalyst to be applied to a transfer hydrogenation reaction, a used solvent does not need to be treated, the reaction undergoes in air, and the catalytic effect is good.
Owner:JIAXING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/f4a96a8b-1fc6-4890-8c89-f72d04e2bc55/HDA0002937573050000011.png)
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/f4a96a8b-1fc6-4890-8c89-f72d04e2bc55/BDA0002937573040000051.png)
![Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane Method for preparing moxifloxacin intermediate (S, S)-2, 8-diazabicyclo [4, 3, 0] nonane](https://images-eureka.patsnap.com/patent_img/f4a96a8b-1fc6-4890-8c89-f72d04e2bc55/BDA0002937573040000061.png)