Method for asymmetric functionalization of nickel-hydrogen catalytic olefin migration promoted by ligand relay strategy
A nickel-hydrogen catalyzed olefin and functionalization technology, applied in organic chemistry methods, chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, etc., can solve problems such as unreported asymmetric hydrogen functionalization, and achieve The effect of reducing difficulty and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
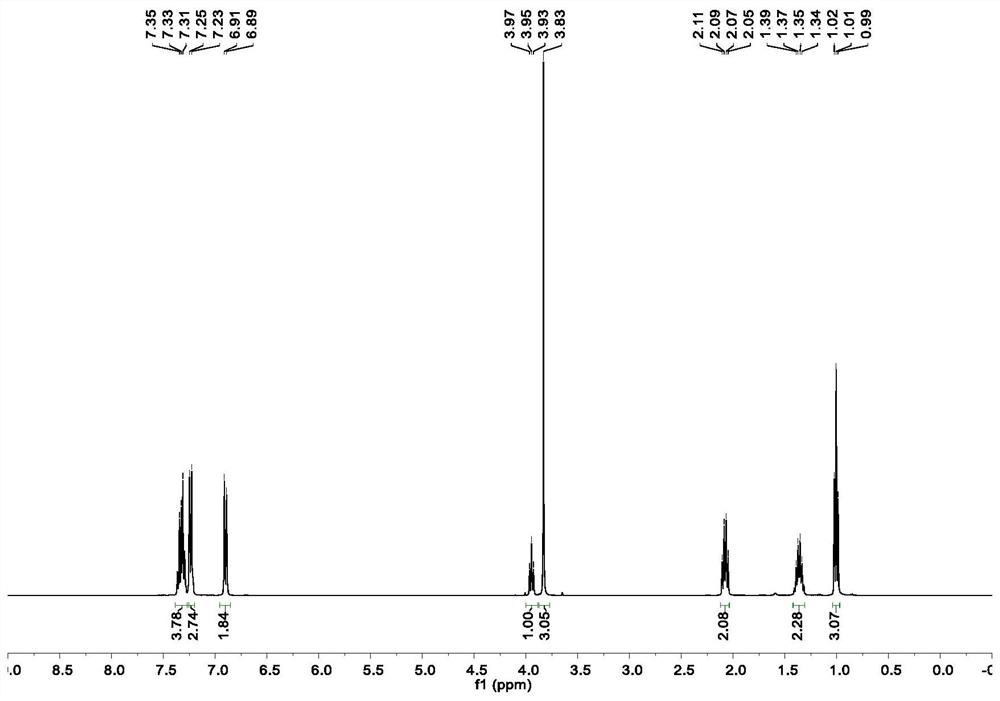
[0045]In a glove box filled with nitrogen, nickel chloride ethylene glycol dimethyl ether complex (2.2mg, 5.0mol%), chiral ligand L26* (7.2mg, 6.0mol%), potassium fluoride (23.2mg ,2.0equiv), achiral ligand L21 [0.21mg, 0.1mL (2.1mg / mL toluene solution)] dissolved in toluene (0.60mL) and DMPU (0.20mL), stirred for 10 minutes and then added the above olefin (30μL , 0.20mmol), 4-iodoanisole (94.0mg, 0.40mmol) and DMMS (49.3μL, 0.40mmol), the reaction tube was sealed and taken out from the glove box, and reacted at 0°C for 24 hours. After the reaction was completed, the reaction solvent was removed by concentration under reduced pressure, and column chromatography separation and purification obtained the target product (colorless oil, yield 75%). 1 H NMR (500MHz, CDCl 3 )δ7.34–7.29(m,2H),7.29–7.25(m,2H),7.20(d,J=8.8Hz,3H),6.87(d,J=8.7Hz,2H),3.91(t,J =7.8Hz, 1H), 3.81(s, 3H), 2.04(q, J=7.8Hz, 2H), 1.37–1.28(m, 2H), 0.97(t, J=7.4Hz, 3H); 13 C NMR (126MHz, CDCl 3 )δ1...
Embodiment 2
[0047]
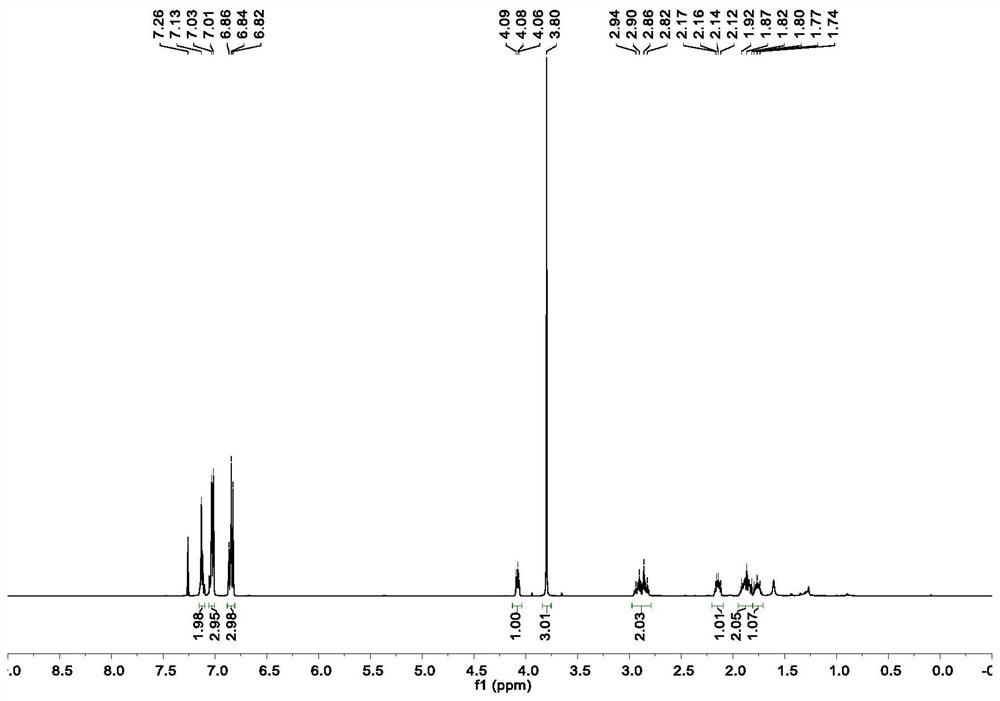
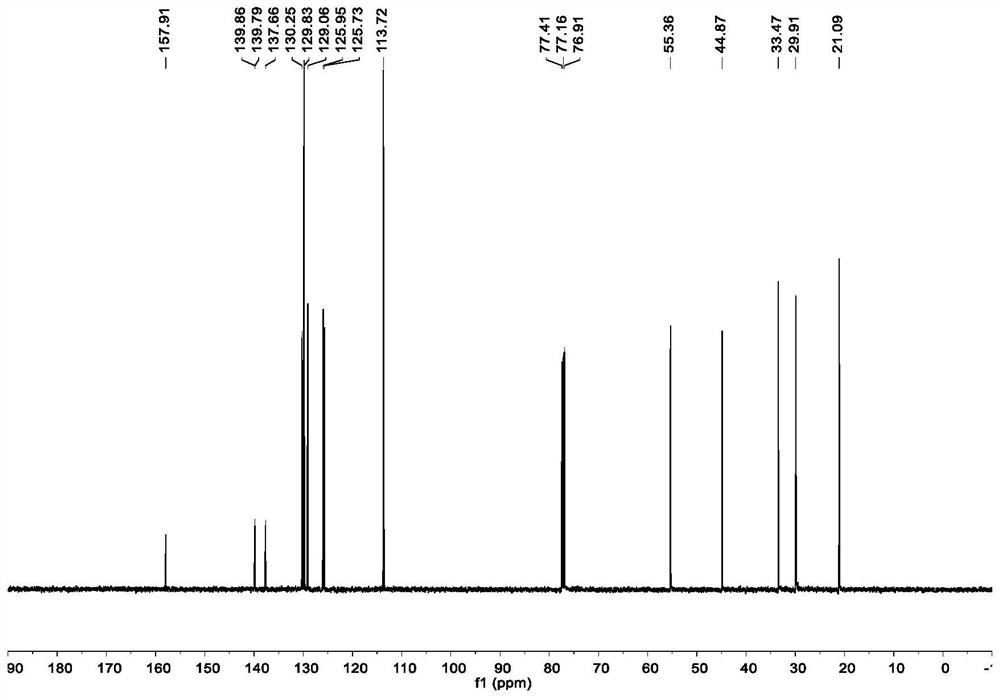
[0048] In a glove box filled with nitrogen, nickel chloride ethylene glycol dimethyl ether complex (2.2mg, 5.0mol%), chiral ligand L26* (7.2mg, 6.0mol%), potassium fluoride (23.2mg ,2.0equiv), achiral ligand L21 [0.42mg, 0.2mL (2.1mg / mL toluene solution)] dissolved in toluene (0.60mL) and DMPU (0.20mL), stirred for 10 minutes and then added the above olefin (26μL , 0.20mmol), 4-iodoanisole (94.0mg, 0.40mmol) and DMMS (49.3μL, 0.40mmol), the reaction tube was sealed and taken out from the glove box, and reacted at room temperature for 24 hours. After the reaction was completed, the reaction solvent was removed by concentration under reduced pressure, and column chromatography separation and purification obtained the target product (colorless oil, yield 73%). 1 H NMR (500MHz, CDCl 3 )δ7.18–7.12(m,2H),7.08–7.02(m,3H),6.90–6.84(m,3H),4.10(t,J=6.7Hz,1H),3.82(s,3H),2.98 –2.84(m,2H),2.21–2.13(m,1H),1.95–1.84(m,2H),1.82–1.74(m,1H); 13 C NMR (126MHz, CDCl 3 )δ157.8, 139...
Embodiment 3
[0050]
[0051] In a glove box filled with nitrogen, nickel chloride ethylene glycol dimethyl ether complex (2.2mg, 5.0mol%), chiral ligand L26* (7.2mg, 6.0mol%), potassium fluoride (23.2mg ,2.0equiv), achiral L21 [0.42mg, 0.2mL (2.1mg / mL toluene solution)] was dissolved in toluene (0.60mL) and DMPU (0.20mL), stirred for 10 minutes and then added the above olefin (32.2mg, 0.20mmol), 4-iodoanisole (94.0mg, 0.40mmol) and DMMS (49.3μL, 0.40mmol), the reaction tube was sealed and taken out from the glove box, and reacted at room temperature for 24 hours. After the reaction was completed, the reaction solvent was removed by concentration under reduced pressure, and column chromatography separation and purification obtained the target product (white solid, yield 81%). 1 H NMR (500MHz, CDCl 3 )δ7.78(d, J=6.9Hz, 2H), 7.49(t, J=7.4Hz, 1H), 7.42(t, J=7.5Hz, 2H), 7.30(d, J=8.6Hz, 2H) ,6.90(d,J=8.6Hz,2H),6.44(d,J=8.2Hz,1H),5.06(q,J=7.6Hz,1H),3.81(s,3H),2.03–1.85(m, 2H), 0.96(t, J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com