Achiral SCS type pincer-like compound and palladium complex thereof
A palladium complex, achiral technology, applied in the field of compound synthesis, can solve problems such as hindering the development of ligands, and achieve the effects of high selectivity, good application prospects, high catalytic activity and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
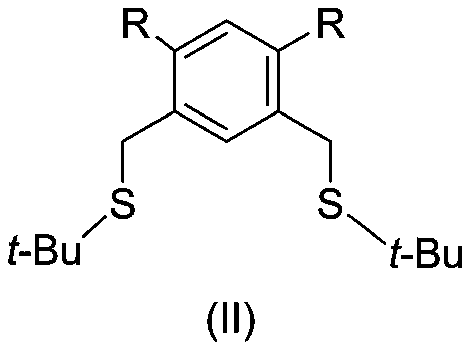
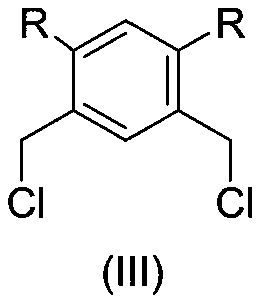
[0061] Compound (Ⅲ) (203.2mg, 1mmol, 1.0eq.), (Ⅳ) (207.5mg 2.3mmol, 1.15eq.), potassium hydroxide (123.4mg, 2.2mmol, 1.1eq.), 15ml anhydrous Ethanol was added into a 100 mL three-neck round bottom flask, and then heated to reflux. TLC detected that the raw material disappeared, and concentrated to dryness to obtain a light yellow crude product. The crude product was dissolved in ethyl acetate and filtered, and the mother liquor was spin-dried to obtain 254.1 mg of white solid product (II), with a yield of 81.8%.
Embodiment 2
[0063]
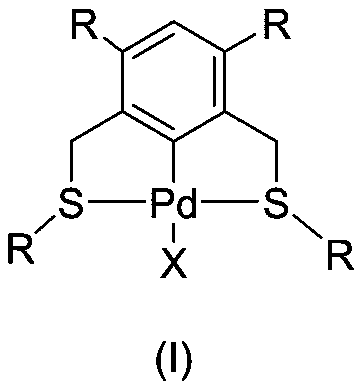
[0064] Add compound (II) (310.6mg, 1mmol, 1.0eq.), bisacetonitrile palladium chloride (272.4mg, 1.05mmol, 1.05eq.) into a 25mL two-necked round-bottomed flask, vacuumize and change nitrogen, and add 10mL of refined Toluene, stirred under reflux for 24 hours, cooled to room temperature, spin-dried the solvent, and purified the product with a 200-300 mesh silica gel column (ethyl acetate / petroleum ether=1 / 20-1 / 3) to obtain 313 mg of a yellow solid (I-A) , yield 69%.
Embodiment 3
[0066]
[0067] A mixture of (I-A) (180.6 mg, 0.4 mmol), KI (1327 mg, 4.0 mmol, 20 equiv.), dichloromethane (9 mL), methanol (6 mL) was stirred in the dark at room temperature. TLC detection until (I-A) disappears. The solvent was spin-dried under reduced pressure, and purified with a 200-300 mesh silica gel column (petroleum ether / ethyl acetate=10 / 1-3 / 1) to obtain 191.1 mg of a yellow solid (I-B), with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com