Preparation method of chiral nanometer copper oxide with optical activity
A nano-copper oxide, optically active technology, used in nanotechnology for materials and surface science, copper oxide/copper hydroxide, nanotechnology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
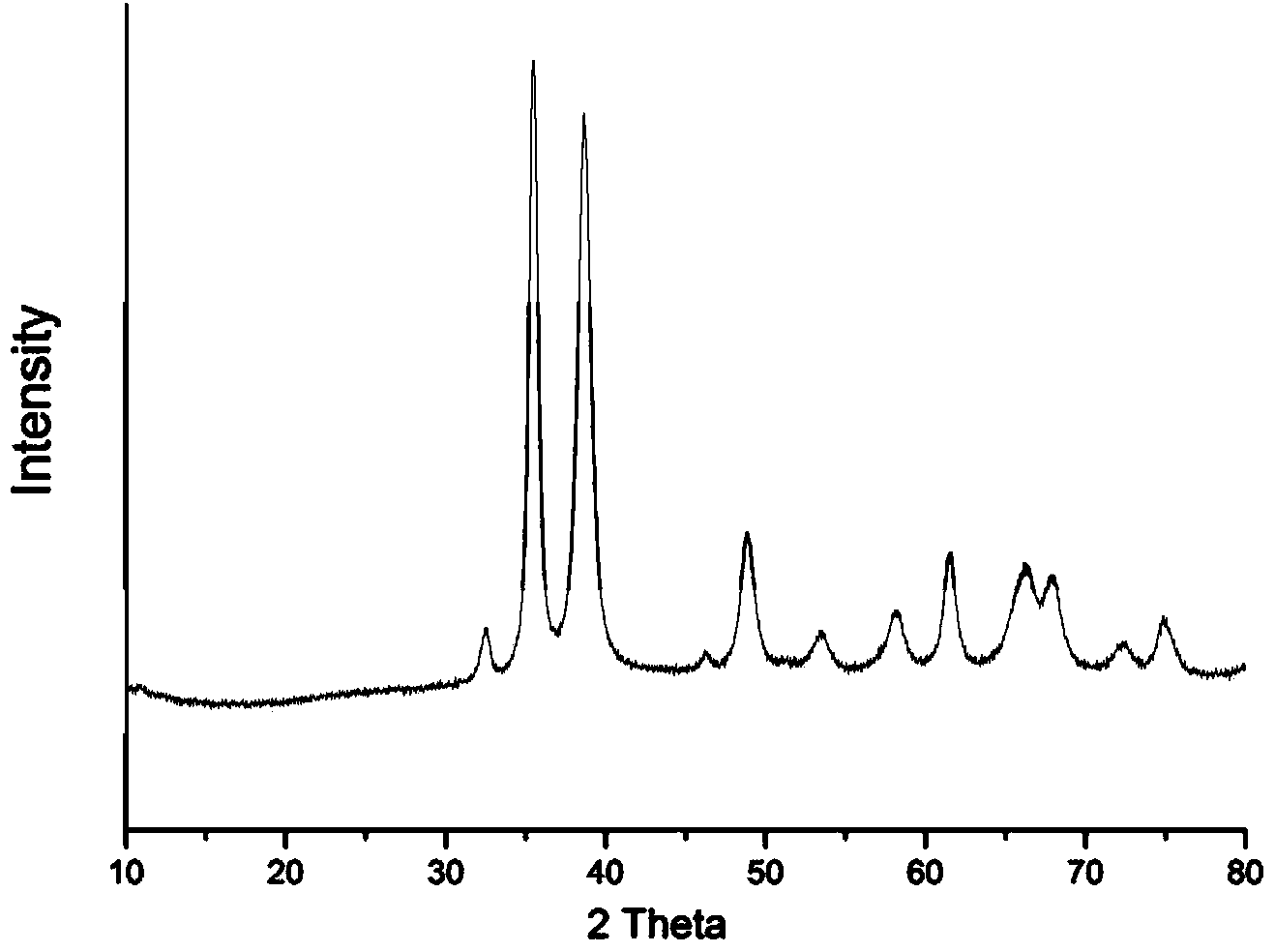
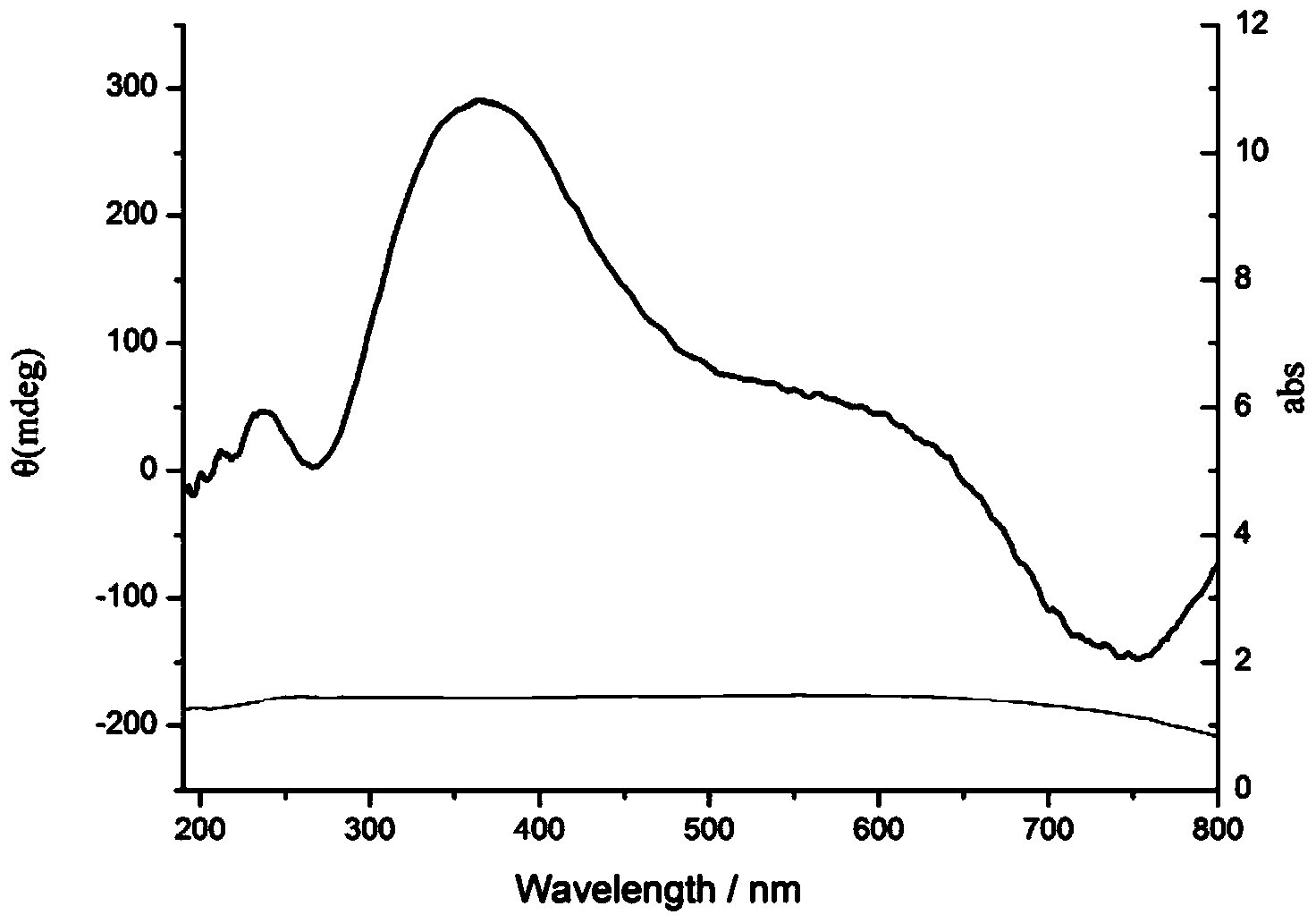
[0023] At room temperature, dissolve 0.288g (1mmol) sodium lauryl sulfate and 0.151g (1mmol) S-phenylalaninol in 25mL deionized water, stir until completely dissolved, add 0.170g (1mmol) copper chloride, stir After reacting for 60 minutes, 15 mL of 4M sodium hydroxide solution was added and reacted at 100° C. for 6 hours. After the reaction, the reaction solution is centrifuged or filtered, washed and dried to obtain optically active chiral nano-copper oxide powder.
[0024] Wherein, in Example 1, in the case of achiral anionic surfactant: chiral small molecule: inorganic copper salt: alkali: water = 1:0.1-2:0.1-3:0.5-200:500-4000 , S-phenylalaninol can be replaced by S(R)-aminopropanol, S(R)-isoleucinol, S(R)-prolinol, S(R)-valinol, S(R)-valinol, (R)-1-amino-2-propanol, (R)-phenylalaninol or S(R)-phenylglycinol; sodium lauryl sulfate can be replaced by sodium tetradecyl carboxylate accordingly , Sodium Cetyl Carboxylate, Sodium Lauryl Sulfate, Sodium Tetradecyl Sulfate, Sod...
Embodiment 2
[0027] At room temperature, dissolve 3.485g (10mmol) sodium dodecylbenzenesulfonate and 0.151g (1mmol) S-phenylalaninol in 90mL deionized water, stir until completely dissolved, then add 0.249g (1mmol) sulfuric acid pentahydrate Copper, after stirring and reacting for 30 minutes, add 1.25mL of 4M sodium hydroxide solution, heat up to 150°C and react for 2 hours. After centrifugation or filtration, washing and drying, optically active chiral nano copper oxide powder is obtained.
[0028] Wherein, in Example 2, in the case of achiral anionic surfactant: chiral small molecule: inorganic copper salt: alkali: water = 1:0.1-2:0.1-3:0.5-200:500-4000 , S-phenylalaninol can be replaced by S(R)-aminopropanol, S(R)-isoleucinol, S(R)-prolinol, S(R)-valinol, S(R)-valinol, (R)-1-amino-2-propanol, (R)-phenylalaninol or S(R)-phenylglycinol; sodium dodecylbenzenesulfonate can be replaced by tetradecylcarboxylate accordingly sodium cetyl carboxylate, sodium lauryl sulfate, sodium tetradecyl s...
Embodiment 3
[0030]At room temperature, dissolve 0.288g (1mmol) sodium lauryl sulfate and 0.206g (2mmol) S-valinol in 72mL deionized water, stir until completely dissolved, then add 0.511g (3mmol) copper chloride, and stir to react After 10 min, 8 g (200 mmol) of sodium hydroxide was added, and the temperature was raised to 180° C. to react for 1 hour. After the reaction, the reaction solution is centrifuged or filtered, washed and dried to obtain optically active chiral nano-copper oxide powder.
[0031] Wherein, in Example 3, in the case of achiral anionic surfactant: chiral small molecule: inorganic copper salt: alkali: water = 1:0.1-2:0.1-3:0.5-200:500-4000 , S-valinol can be replaced by S(R)-aminopropanol, S(R)-isoleucinol, S(R)-prolinol, (R)-valinol, S(R) )-1-amino-2-propanol, S(R)-phenylalaninol or S(R)-phenylglycinol; sodium lauryl sulfate can be replaced by sodium tetradecyl carboxylate, Sodium Cetyl Carboxylate, Sodium Lauryl Sulfate, Sodium Tetradecyl Sulfate, Sodium Cetyl Sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com