Method for preparing Alpha-methyl-Alpha, Alpha-disubstituted-Alpha-aminophenol and derivatives thereof
A disubstituted, amino acid technology, applied in the preparation of carbamic acid derivatives, organic compounds, chemical instruments and methods, etc., can solve the problems of increased synthesis costs, long synthesis steps, low optical purity, etc., and achieve easy operation and Effects of scale-up, mild reaction conditions, and ease of purchase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
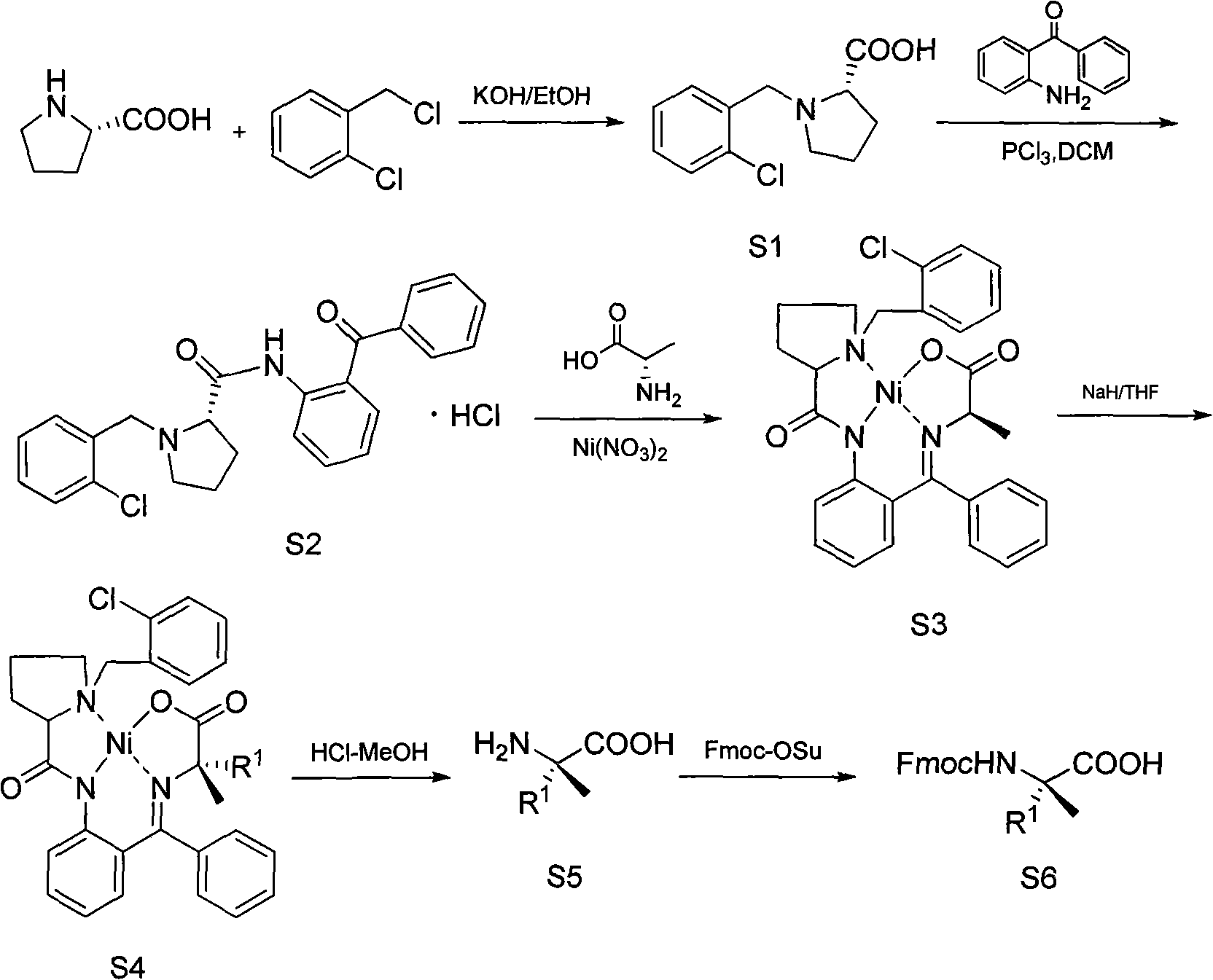
[0059] Example 1: Synthesis of (S)-α-allyl-Fmoc-alanine (R 1 = allyl)
[0060] 1. Synthesis of S1
[0061] 700 mL of isopropanol was heated to 55° C., 145.9 g of potassium hydroxide was added, and after the dissolution was complete, 100 g of L-proline was added. After complete dissolution, 167.5 g of o-chlorobenzyl chloride was added. After stirring evenly, thin layer chromatography showed that the reaction of L-proline had been completed. Adjust the pH of the system to 5-6 with 6N hydrochloric acid, add dichloromethane and let stand for 4 hours, then filter, wash the filter cake with dichloromethane, collect the filtrate, spin dry, and crystallize with acetone. 185.37 g of S1 was precipitated as a white solid with a yield of 89%. H NMR spectroscopy 1 H NMR (CD 3 OD), δ: 2.08-2.80 (m, 4H,); 3.48 (m, 1H); 3.77 (m, 1H,); 4.20 (m, 1H); 4.67, 4.51 (AB, J=13.1Hz, 2H, ); 7.66-8.00 (m, 3H).
[0062] 2. Synthesis of S2
[0063] Dissolve 31.0 g of S1 in 270 mL of dichlorometha...
Embodiment 2
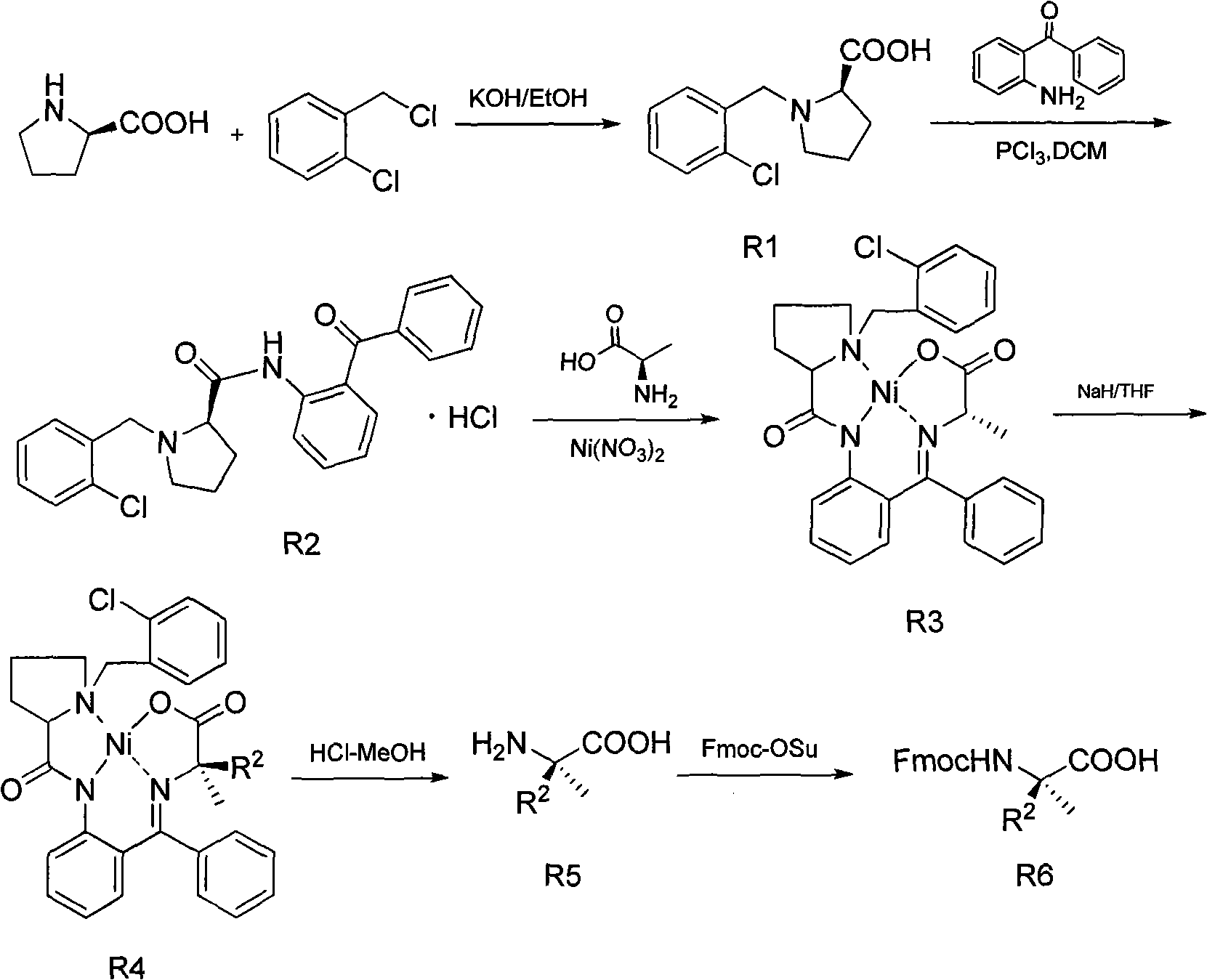
[0072] Embodiment 2: the synthesis of (R)-alpha-(5-pentenyl)-Fmoc-alanine (R 2 =5-pentenyl)
[0073] 1. Synthesis of R1
[0074] Heat 350mL of isopropanol to 55°C, add 73g of potassium hydroxide, and after the dissolution is complete, add 50g of D-proline. Add 84 grams of o-chlorobenzyl chloride after complete dissolution. After stirring evenly, thin-layer chromatography showed that L-proline had reacted completely. Use 6N hydrochloric acid to adjust the pH of the system to 5-6, add dichloromethane and let stand for 4 hours, then filter, wash the filter cake with dichloromethane, collect the filtrate and spin it dry, and crystallize with acetone. 78.1 g of R1 precipitated as a white solid, yield 75%. H NMR spectrum 1 H NMR (CD 3 OD), δ: 2.08-2.80 (m, 4H,); 3.48 (m, 1H); 3.77 (m, 1H); 4.20 (m, 1H); 4.67, 4.51 (AB, J=13.1Hz, 2H,) ; 7.66-8.00 (m, 3H).
[0075] 2. Synthesis of R2
[0076] Dissolve 46.5 grams of R1 in 400 mL of dichloromethane, place in an ice bath, and sl...
Embodiment 3
[0085] Embodiment 3: the synthesis of (S)-alpha-benzyl-Fmoc-alanine (R 1 = benzyl)
[0086] The synthesis of S1, S2 and S3 in this example is the same as Example 1.
[0087] 1. Synthesis of S4
[0088] Add 6.54 g of S3 to a 100 mL three-necked flask in sequence, distill 40 mL of tetrahydrofuran, place the reaction system in an ice bath, cool to 0°C, add 1.8 g of sodium iodide and 0.87 g of sodium hydride under the protection of argon, and stir for 1 hour Afterwards, 5.12 g of benzyl bromide was added dropwise at 0°C, and reacted at 0-10°C for 5 hours. Thin-layer chromatography showed that the reaction of the raw materials was complete, and the reaction was quenched with ice, neutralized with acetic acid, extracted with dichloromethane (50 mL×3), and the organic phases were combined and dried overnight with anhydrous magnesium sulfate. After filtration, the filtrate was collected, and the obtained crude product was spin-dried with ethyl acetate as eluent, and silica gel colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com