Single chiral center catalyst, preparation method thereof and method for catalytically synthesizing chiral alcohol compounds and chiral alpha-allyl alcohol
A chiral center and catalyst technology, which is applied in the field of catalytic synthesis of chiral alcohol compounds and chiral α-allyl alcohol, can solve the problems of large substrate scope limitation, low substrate universality, and large limitations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] The preparation method of a class of monochiral center catalysts according to an embodiment is a preparation method of the single chiral center catalysts of the above-mentioned embodiments, comprising the following steps:
[0088] reacting the precursor with the first ligand in the first solvent to prepare the intermediate;
[0089] The catalyst is prepared by reacting the intermediate with the second ligand in a second solvent.
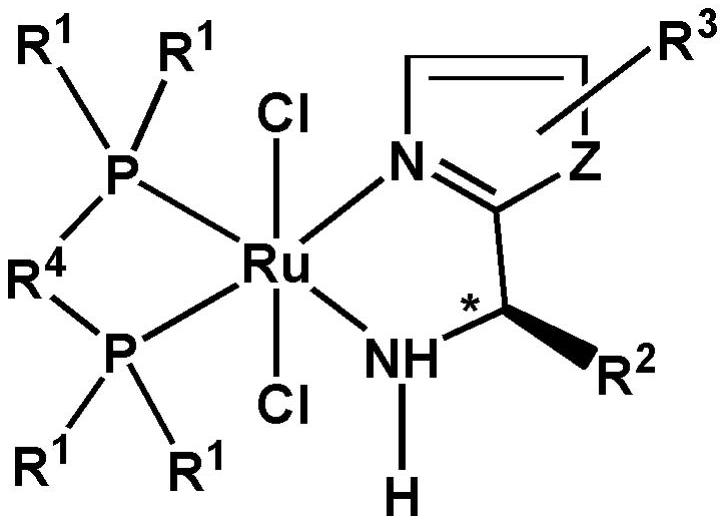
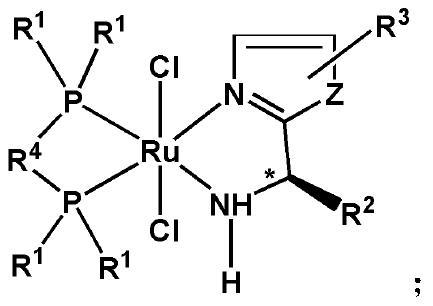
[0090] Wherein, the precursor is tris(triphenylphosphorus) ruthenium dichloride. Selecting the above precursors can improve the activity of the reaction.
[0091] The first ligand has the following general formula: In the formula, Z is -S-, -N=CH- or -CH=CH-. Specifically, the first ligand has the following general formula:
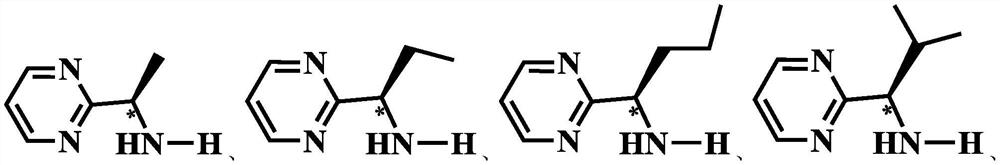
[0092] In the formula, R 2 One selected from a chain alkyl group and a cycloalkane group. Further, R 2 from C 1 ~C 4 The chain alkyl and C 3 ~C 6 one of the cycloalkyl groups. Preferably, R 2 from -CH 3 ,...
Embodiment 1-1
[0172] The preparation process of the monochiral center catalyst of the present embodiment is as follows:
[0173] (1) Under the protection of argon, the molar ratio of tris (triphenylphosphine) ruthenium (II) dichloride and isopropyl substituted 2-(aminomethyl) chiral pyrimidine ligand is 1:1. It was dissolved in dichloromethane, and the reaction was stirred at 25° C. for 12 h to obtain the first reaction solution. The first reaction solution is filtered to obtain a first filtrate, the first filtrate is subjected to vacuum distillation to obtain a first solid, the first solid is dissolved in dichloromethane, and n-hexane is added thereto for recrystallization to obtain an intermediate, The volume ratio of dichloromethane to n-hexane was 1:1.
[0174] (2) Combine the intermediate obtained above with 1,2-bis(diphenylphosphino)propane It was dissolved in toluene and stirred at 120° C. for 12 h to obtain a second reaction solution, wherein the molar ratio of the intermediate ...
Embodiment 1-2
[0182] The preparation process of the monochiral center catalyst of the present embodiment is as follows:
[0183] (1) Under the protection of argon, the molar ratio of tris(triphenylphosphine) ruthenium(II) dichloride and isopropyl substituted 2-(aminomethyl) chiral pyridine ligand is 1:1. Dissolved in dichloromethane, and then stirred at 25 ° C for 12 hours to obtain the first reaction solution, the first reaction solution was filtered to obtain the first filtrate, the first filtrate was distilled under reduced pressure to obtain the first solid, and the first After the solid was dissolved in dichloromethane, n-hexane was added thereto for recrystallization to obtain an intermediate, and the volume ratio of dichloromethane and n-hexane was 1:1.
[0184] (2) Combine the intermediate obtained above with (1,2-bis(3,5-di-tert-butylphenyl)-diphenylphosphino)benzene It was dissolved in toluene and stirred at 120 °C for 12 h to obtain a second reaction solution, in which the int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com