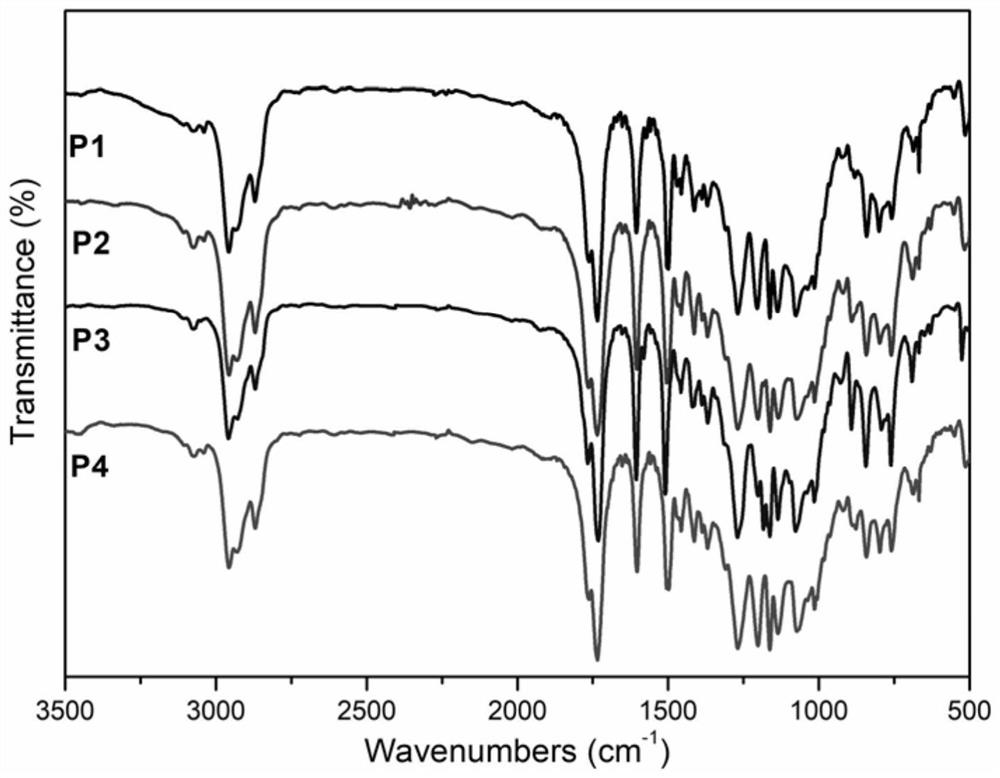
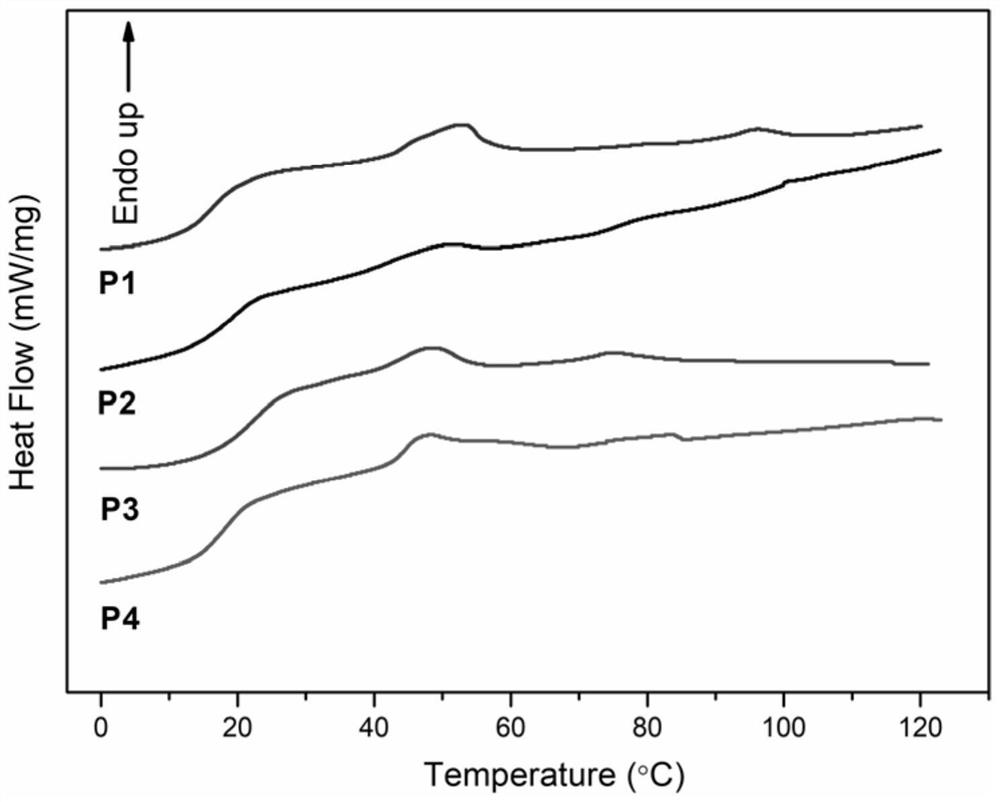
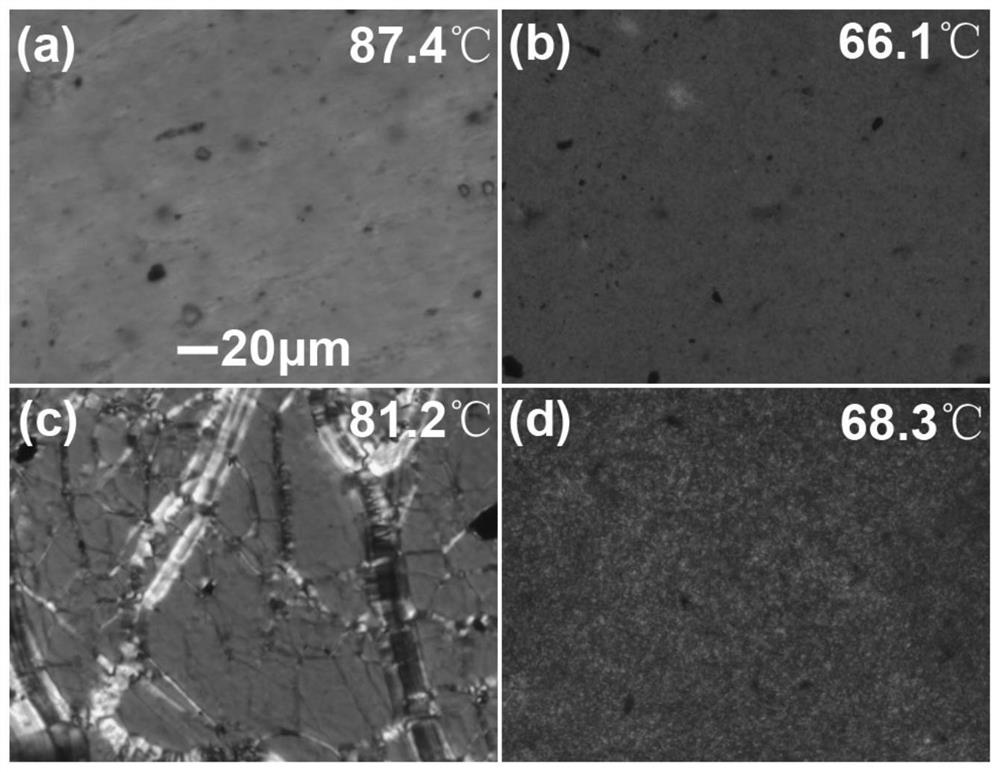
Chiral menthyl liquid crystal oligomer film and preparation method thereof
A mint-based, oligomer technology, applied in the direction of liquid crystal materials, chemical instruments and methods, etc., can solve the problems of not being able to retain colors, etc., and achieve the effects of easy industrial production, high yield, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Step 1, preparing a chiral menthyl liquid crystal monomer.
[0043] Add 0.10 mol of adipic acid to a 150 mL single-necked bottle, add 30 mL of thionyl chloride, heat up to 64°C, heat to reflux for 6 hours, and distill under reduced pressure to obtain Intermediate 1 after the reaction is complete.
[0044] Move Intermediate 1 to a 250 mL three-neck flask, add 150 mL of chloroform as a solvent, heat to reflux, slowly add 0.10 mol of menthol chloroform solution dropwise, and heat to reflux for 6 hours. After the reaction is completed, filter under reduced pressure to obtain intermediate Body 2.
[0045] Add 0.12mol of p-hydroxybenzoic acid, 200mL of tetrahydrofuran and 0.1mol of pyridine into a 500mL three-neck flask, heat to reflux, slowly add the above intermediate 2 dropwise, and heat to reflux for 6h. After the reaction, cool and filter under reduced pressure, spin the filtrate to dryness, add dichloromethane, heat to reflux for 0.5h, cool and filter. The dichloromet...
Embodiment 2
[0067] Polymethyl hydrogen-containing linear siloxane PMHS, chiral menthyl liquid crystal monomer (M 1 ), (E)-1-(4-(2-(4-(allylcyclohexyl)vinyl)cyclohexyl)-4-methoxybenzene (M 2 ) was dissolved in 50mL xylene, stirred, and 0.8mL tetrahydrofuran solution of hexachloroplatinic acid (5mg·mL -1 ), N 2 Under protection, the temperature was raised to 65°C, and the reaction was carried out for 10 hours. After the reaction, the reaction solution was cooled to room temperature, poured into a beaker filled with about 100mL of methanol, left to stand overnight to precipitate a precipitate, filtered with suction, recrystallized from the filter cake with methanol, and dried to obtain a chiral menthyl liquid crystal oligomer, see Formula VI.
[0068]
[0069] Prepare O1-O8 series of chiral mint-based liquid crystal oligomers according to the above method, and the feed ratio is shown in Table 2 below:
[0070] Table 2 Feed ratio of chiral menthol-based liquid crystal oligomers
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com