Achiral side methyl alkyl terphenyl liquid crystal compound, production method, liquid crystal composition and application
A technology for methyl alkyl terphenyls and liquid crystal compounds, which is applied in the field of achiral side methyl alkyl terphenyl liquid crystal compounds and their preparation, can solve the problem that liquid crystal materials for microwaves cannot meet the requirements, and the low temperature performance of liquid crystal materials is affected. The problem of large dielectric loss of liquid crystal materials can increase the microwave phase modulation capability, increase the optical anisotropy, and reduce the effect of resonance absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
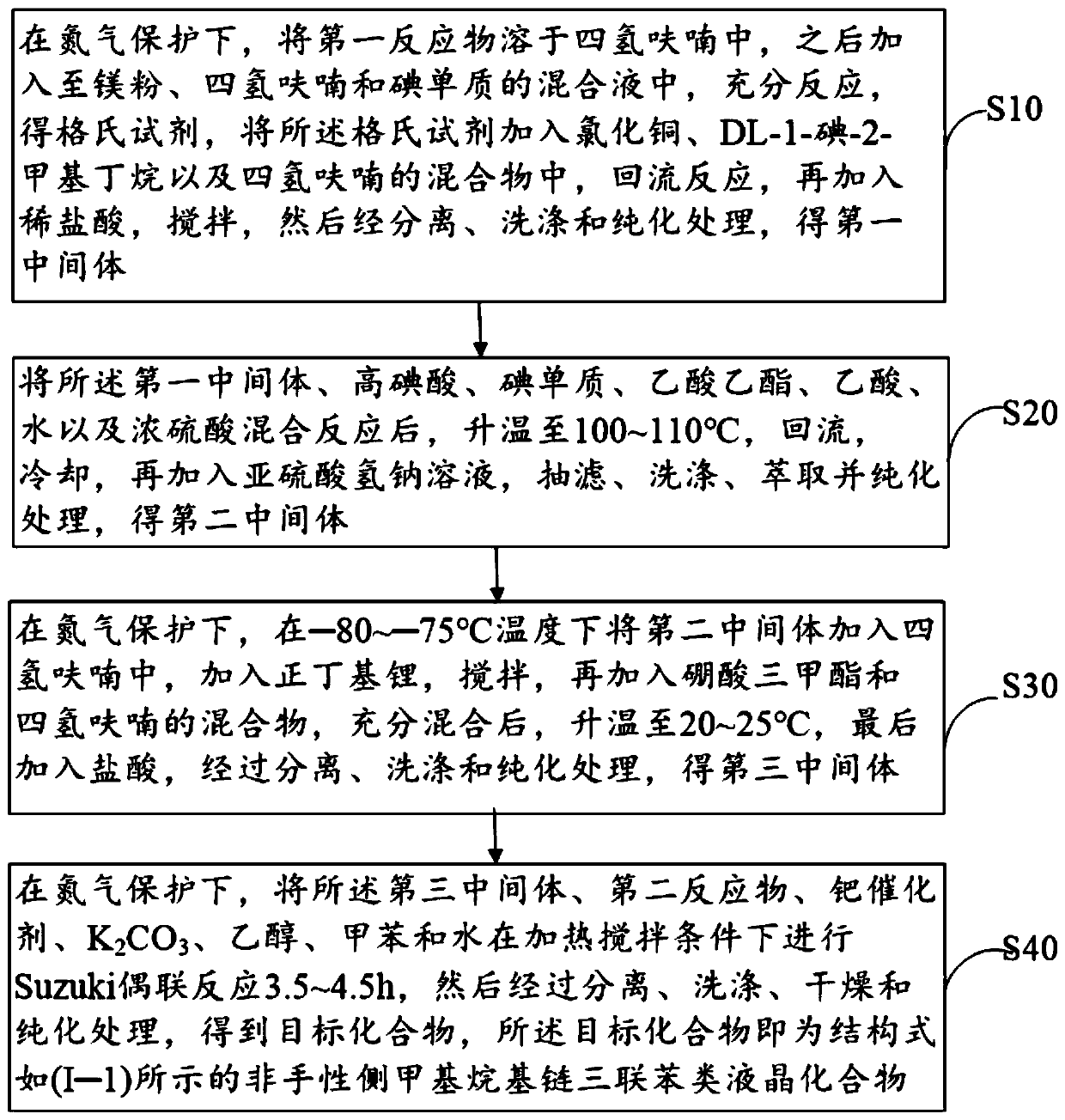
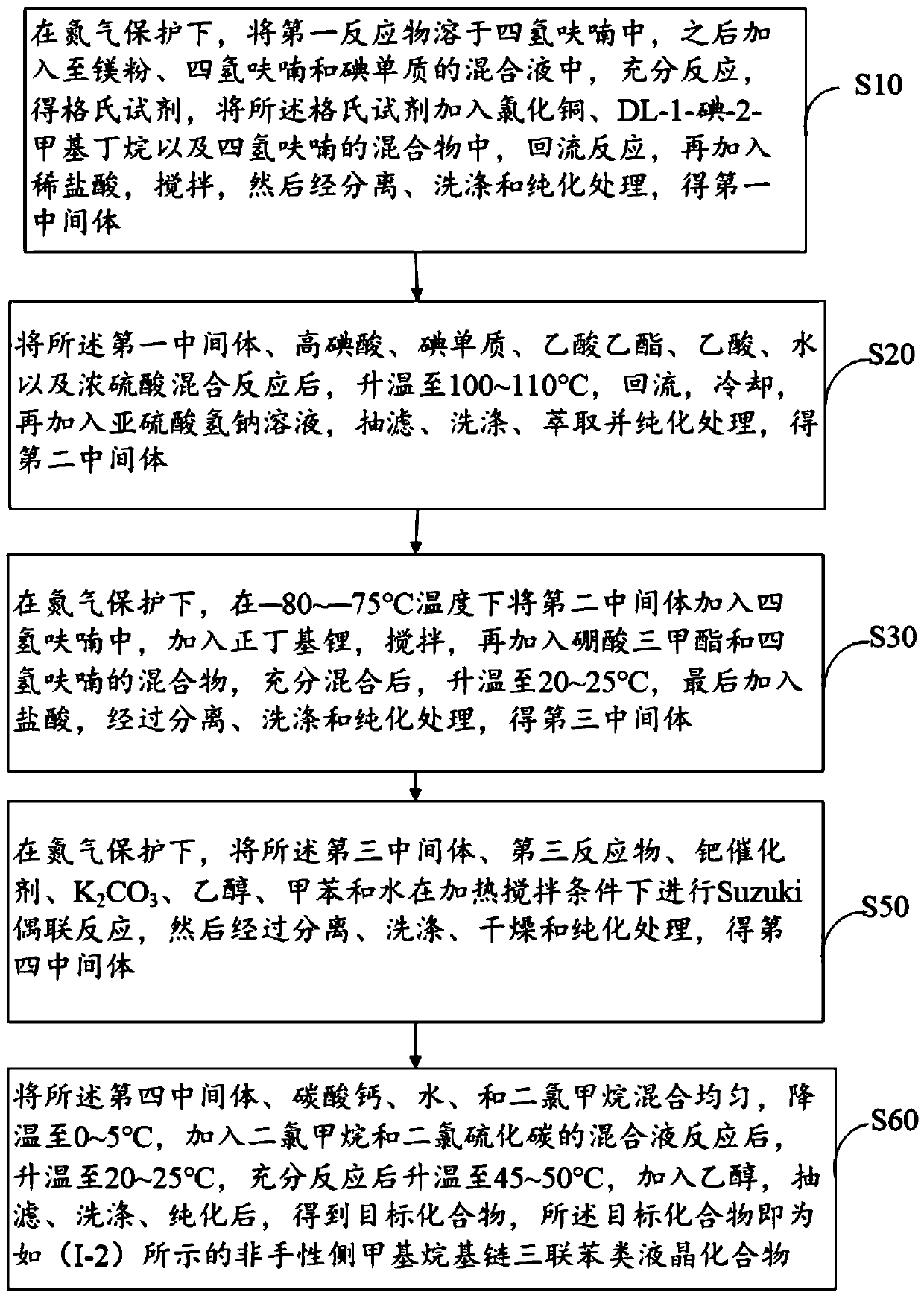
[0065] figure 1 A schematic flow diagram of an embodiment of the preparation method of the achiral pendant methyl alkyl terphenyl liquid crystal compound provided by the present invention, including the following steps:
[0066] Step S10: under the protection of nitrogen, dissolve the first reactant in tetrahydrofuran, then add it to the mixture of magnesium powder, tetrahydrofuran and iodine, and react fully to obtain a Grignard reagent, which is added to the chlorinated In the mixture of copper, DL-1-iodo-2-methylbutane and tetrahydrofuran, reflux reaction, then add dilute hydrochloric acid, stir, and then separate, wash and purify to obtain the first intermediate;
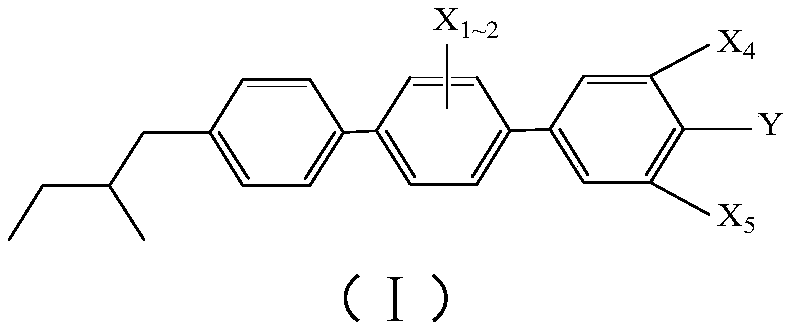
[0067] Wherein, the first reactant in step S10 is a compound having the structure shown in the following structural formula (II):
[0068]
[0069] Wherein, X in structural formula (II) 1 and x 2 each independently selected from H atom, F atom, Cl atom or -CH 3 .
[0070] In this step, separation, washin...
Embodiment 1
[0119] Example 1 Synthesis of achiral pendant methyl alkyl terphenyl liquid crystal compound 4(1) PPUS, the structural formula is:
[0120]
[0121] The preparation process is:
[0122] (1) In a 500mL three-necked flask equipped with magnetic stirring, add 3g (0.124mol) of magnesium chips, 10mL of tetrahydrofuran and 1-2 iodine simple substances, and then dissolve 26.7g (0.115mol) of bromine in 100mL of anhydrous tetrahydrofuran The biphenyl solution was added to a 250ml constant pressure dropping funnel. Under nitrogen protection, add a little bromobenzene dropwise from the constant pressure dropping funnel into the three-necked flask, and heat slightly. After the reaction was initiated, the heating was stopped. Stir slowly, and at the same time, drop bromobenzene into the three-necked flask at a constant speed to make the reflux stable. After dropping, heat up and reflux for 30 minutes until the magnesium is nearly completely reacted, stop heating, and cool to room tem...
Embodiment 2
[0132] Example 2 Synthesis of achiral pendant methyl alkyl terphenyl liquid crystal compound 4 (1) PPGS, the structural formula is:
[0133]
[0134] The steps are the same as in Example 1, except that the 2,6-difluoro-4-bromoaniline in step (4) is replaced by 2-fluoro-4-bromoaniline; the temperature is raised to 100°C in step (2) , in step (3), cool down to -80°C in a low-temperature tank, heat up to 25°C after stirring for 2 hours, control the reaction temperature at 40°C in step (4), cool down to 5°C in an ice bath in step (5), and then naturally The temperature was raised to 25°C, and then to 50°C to continue the reaction for 50 minutes.
[0135] Detection by hydrogen-nuclear magnetic resonance spectrum and fluorine-nuclear magnetic resonance spectrum proves that the structure of the prepared target compound is consistent with the structure shown in the above structural formula.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com