Chiral MOFs material and application of chiral MOFs material as chromatographic stationary phase in resolution of chiral drugs
A chiral, solution technology, applied in other chemical processes, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of high back pressure and poor separation performance, achieve uniform particles, good dispersion, and ensure column efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
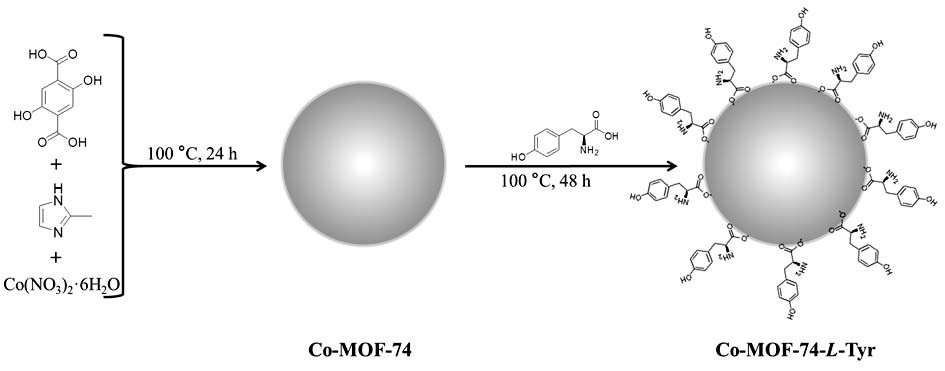
[0033] Example 1 Preparation of chiral MOFs material of the present invention
[0034] (1), the chiral MOFs material of the present invention, its synthetic method comprises the following steps:
[0035]In the first step, N,N-dimethylamide, methanol and water are mixed together at a volume ratio of 1:1:1 to obtain a mixed reagent; then 2.5 mmol 2-methylimidazole (ie 2-MI) is dissolved 2-Methylimidazole solution was obtained in 30 mL mixed reagent, and 0.5 mmol 2,5-dihydroxyterephthalic acid (i.e. H 4 dhtp) was dissolved in 30 mL mixed reagent to obtain 2,5-dihydroxyterephthalic acid solution, and 2 mmol cobalt nitrate hexahydrate was dissolved in 40 mL mixed reagent to obtain cobalt nitrate hexahydrate solution;
[0036] In the second step, the 2-methylimidazole solution prepared in the first step is slowly added dropwise to the cobalt nitrate hexahydrate solution, during which the color of the solution gradually changes from wine red to purple; Add 2,5-dihydroxyterephthalic...
Embodiment 2
[0064] Example 2 Application of Chiral MOFs Materials as Stationary Phases in Resolving Chiral Drugs
[0065] The chiral MOFs material prepared in Example 1 was used as the stationary phase of high performance liquid chromatography and packed in a chromatographic column (i.e., column A in Table 2), with n-hexane / isopropanol as the mobile phase, and the column temperature was 25°C C, the detection wavelength is 254 nm, and the enantiomers of the following chiral drugs are resolved: nitrendipine, nimodipine, binaphthol, benzoin, naphthalene ethanol, bifurfural, flavanone and HR-8, see the structural formulas in order Figure 8 (a)-(h) in. Among them, the chromatographic conditions and separation properties of the above eight chiral drugs and drug intermediates are shown in Table 2.
[0066] Table 2 Resolution results of chiral columns of the present invention and commercially available chiral columns for chiral drugs and intermediates
[0067]
[0068] Note: Column A in Tab...
Embodiment 3
[0071] Example 3 The Co-MOF-74- L -Study on the repeatability of Tyr as a chiral column
[0072] The chiral MOFs material prepared in Example 1 is packed in a chromatographic column as a stationary phase of high performance liquid chromatography, with n-hexane / isopropanol as mobile phase, column temperature 25 ° C, detection wavelength 254 nm, repeat Nimodipine and flavanones were resolved (see Table 2 for the mobile phase of nimodipine and flavanones), and the resolution results of nimodipine are shown in Figure 10-1 , see 10-2 for the resolution results of flavanones, where Figure 10-1 and Figure 10-2 (1)-(5) correspond to the 1st stitch, 5th stitch, 10th stitch, 20th stitch and 50th stitch respectively.
[0073] From Figure 10-1 It can be seen that the peak eluting time and resolution of the 1st and 50th needles of nimodipine are basically consistent, and the relative standard deviation (RSD, n=5) of the resolution of the five injections is calculated based on the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| separation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com