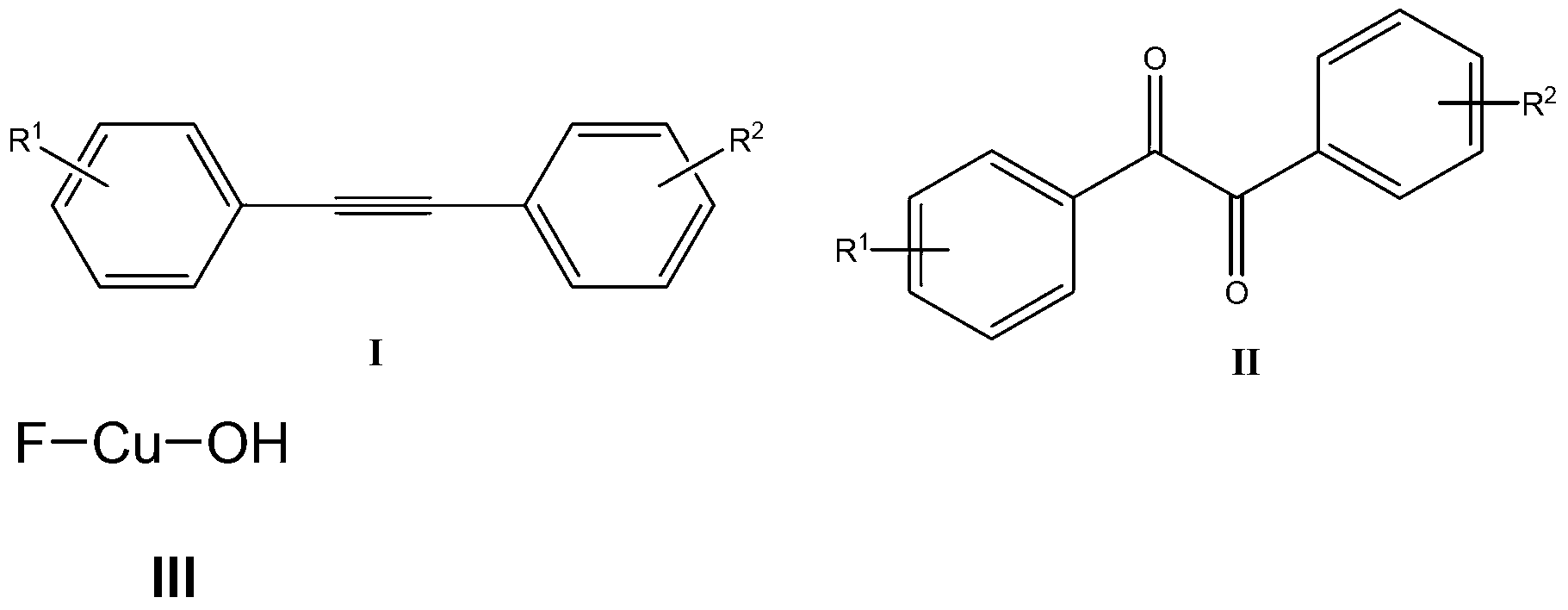
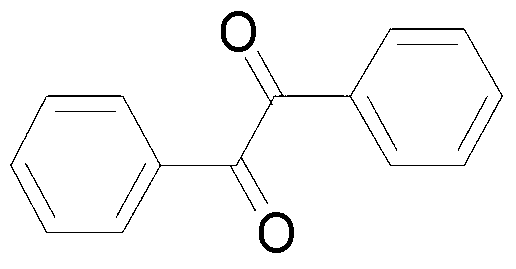
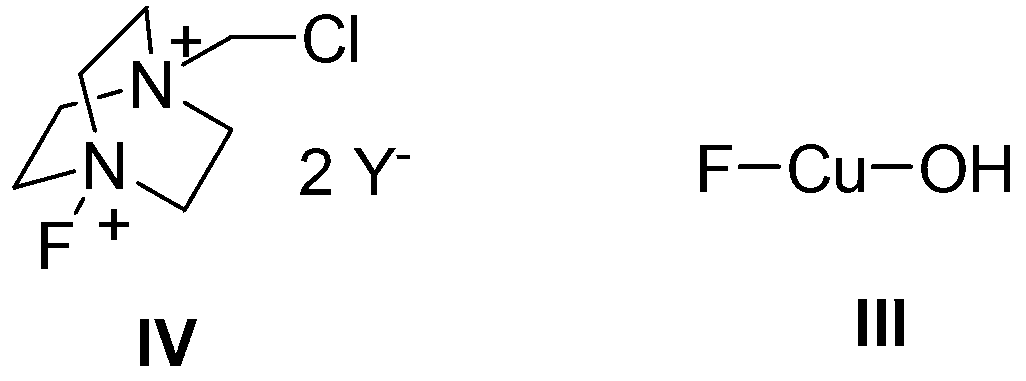Method for catalyzing and synthesizing benzil derivatives from alkali type copper fluoride
A technology of copper fluoride and compounds, which is applied in the field of benzil derivatives catalyzed by basic copper fluoride, can solve the problems of complicated preparation steps, difficult separation and purification, harsh temperature, etc., and achieves mild reaction conditions, simple operation, The effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] (1) Mix Cu powder (64mg, 1mmol), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane di(tetrafluoroborate) salt (Selectfluor, 354.3mg, 1mmol), was added to a 200mL round bottom flask, and then 10mL of acetonitrile / water (V:V=50:1) was added as a solvent. Then, magnetically stirred at room temperature for 1 h to obtain a light blue solution and a white precipitate. Filter to remove the white precipitate, concentrate the mother liquor, and precipitate 75 mg of basic copper fluoride, with a yield of 75%.
[0035] Characterization data of FCuOH: IR:ν3150(OH)cm -1 ; 19 F NMR (376MHz, CD 3 CN):δ-151.06ppm;ESI-MS:m / z:80[M-F] + .
[0036] (2) Add 35.6mg (0.2mmol) toluene, 141.7mg (0.4mmol) Selectfluor, 1mg (0.01mmol) FCuOH to a 10mL round bottom flask, then add 2mL acetonitrile / water (V:V=50:1 ) as a solvent. Then, the mixture was magnetically stirred for 4 h at room temperature. Then, 10 g of column chromatography silica gel (100-200 mesh) was added to th...
Embodiment 2
[0039] In step (1), Selectfluor was changed to 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane di(hexafluorophosphate) salt (470.6mg, 1mmol), and other operations were the same Example 1, 74 mg of basic copper fluoride was obtained, with a yield of 74%.
[0040] In step (2), the amount of basic copper fluoride was changed to 0.6 mg (0.006 mmol), and the other operations were the same as in Example 1 to obtain benzil with a yield of 77%.
Embodiment 3
[0042] In step (1), Selectfluor was changed to 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane di(trifluoromethanesulfonate) salt (478.8mg, 1mmol), others The operation was the same as in Example 1, and 72 mg of basic copper fluoride was obtained, with a yield of 72%.
[0043] In step (2), the amount of basic copper fluoride was changed to 2 mg (0.02 mmol), and other operations were the same as in Example 1 to obtain benzil with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com