Patents
Literature
381 results about "Chrysene" patented technology
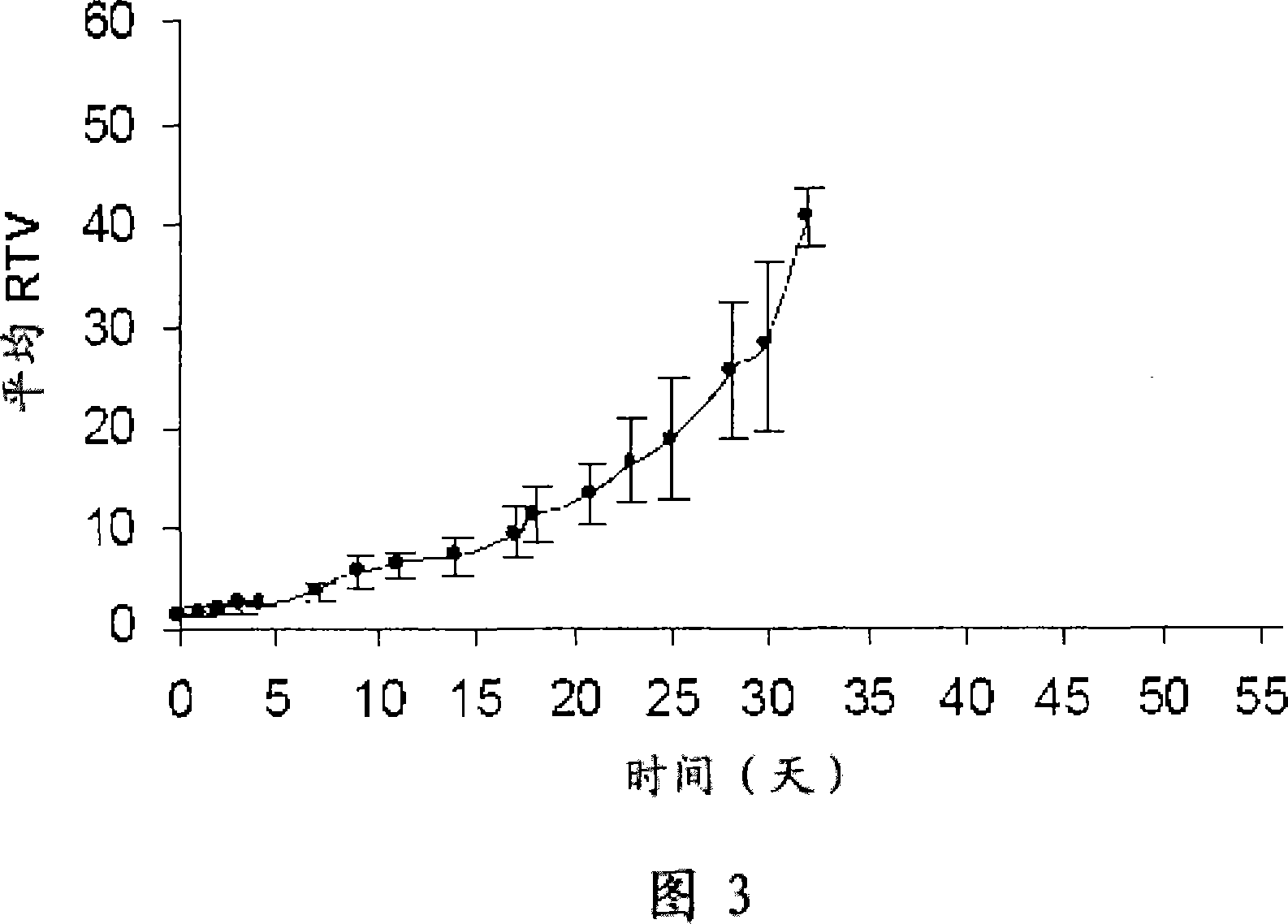
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C₁₈H₁₂ that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5-6 mg/kg.
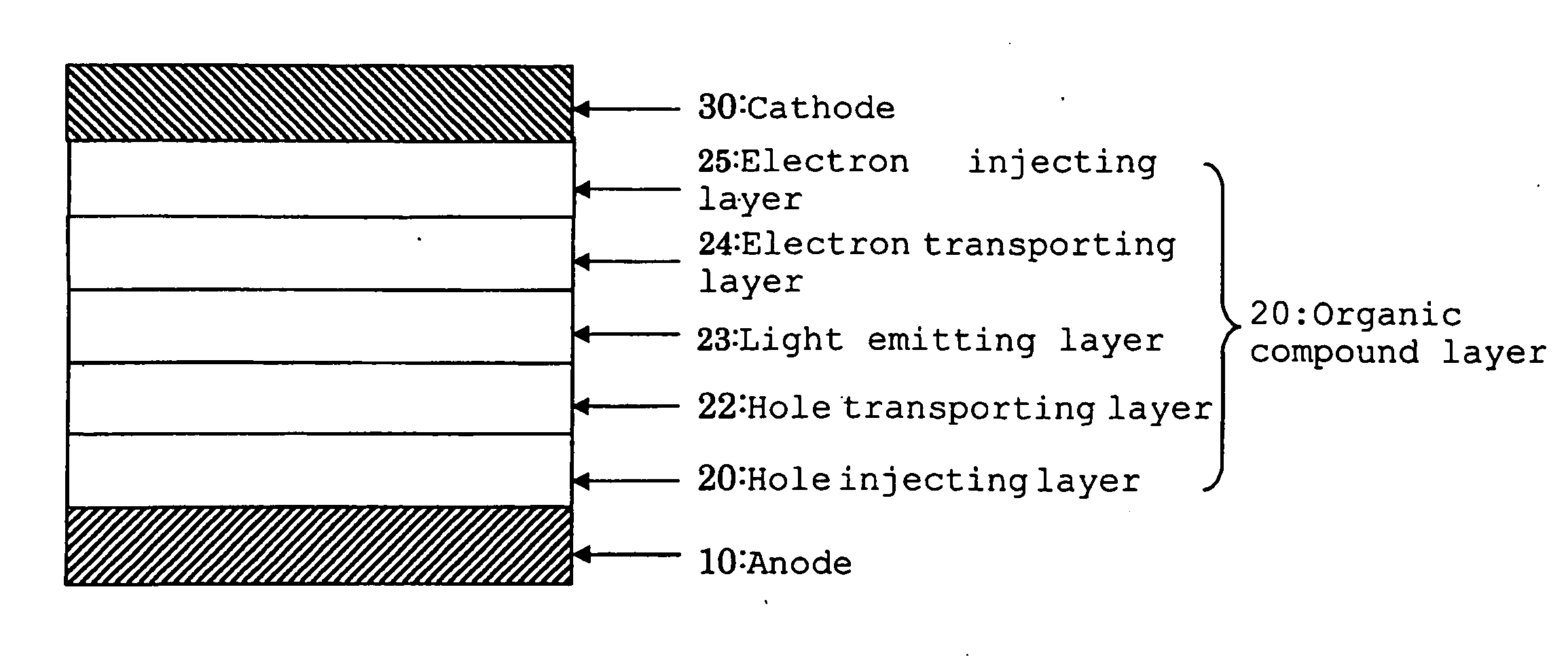
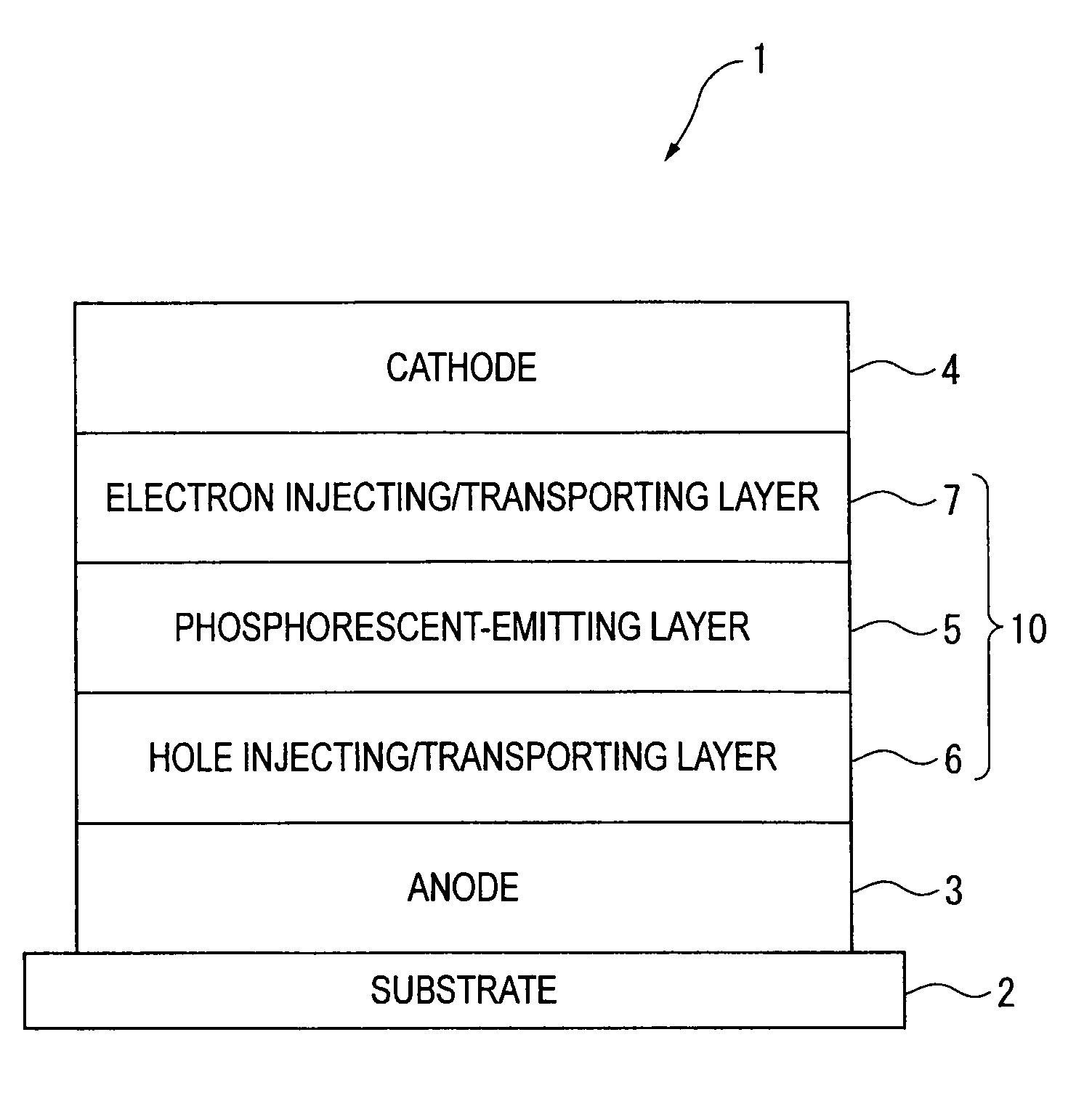
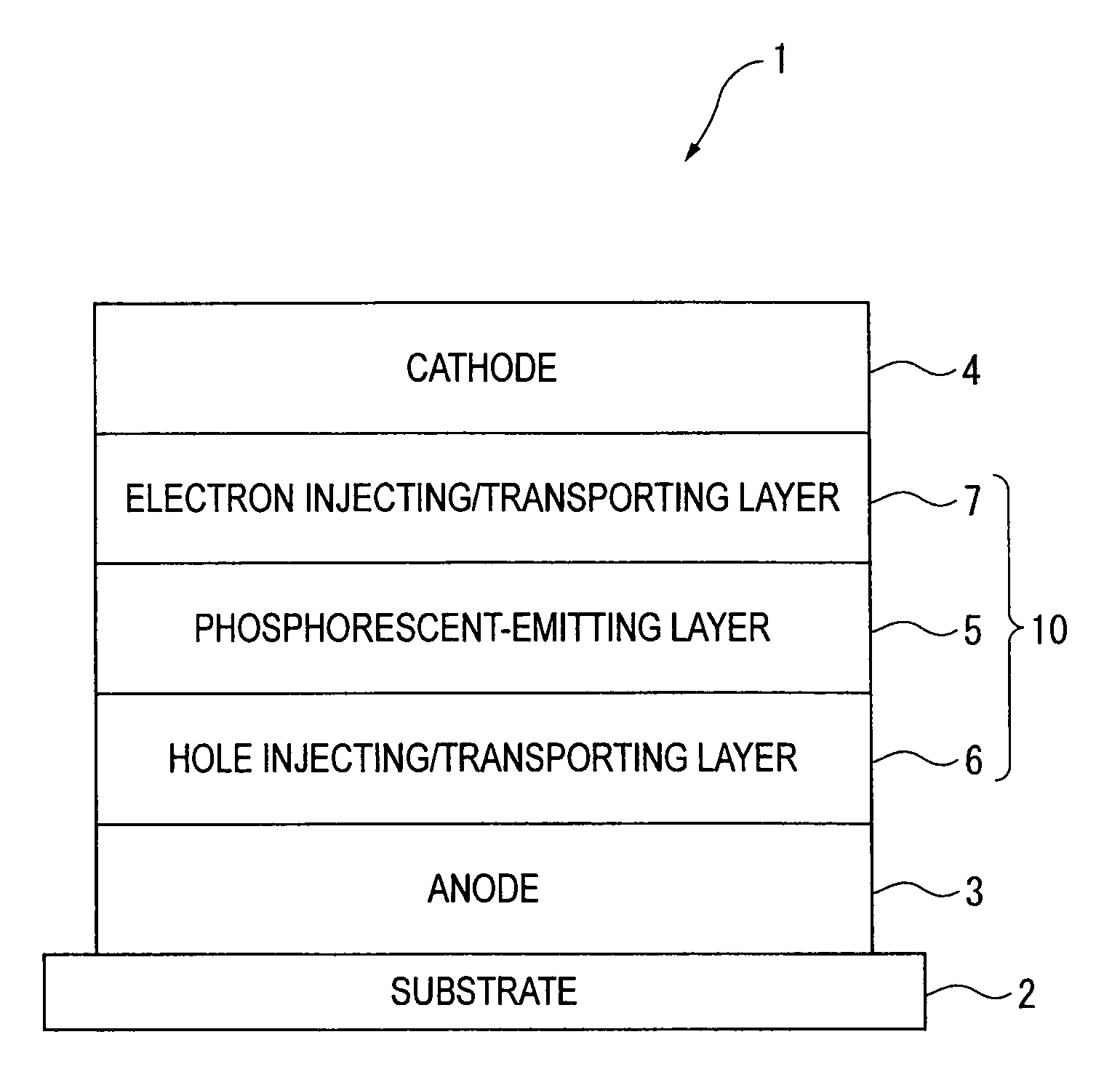
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090009065A1Improve efficiencyLong lastingSilicon organic compoundsDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
Owner:IDEMITSU KOSAN CO LTD
Organic electroluminescence device and material for organic electroluminescence device
InactiveUS20090045731A1Improve efficiencyLong life-timeDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmFluoranthene
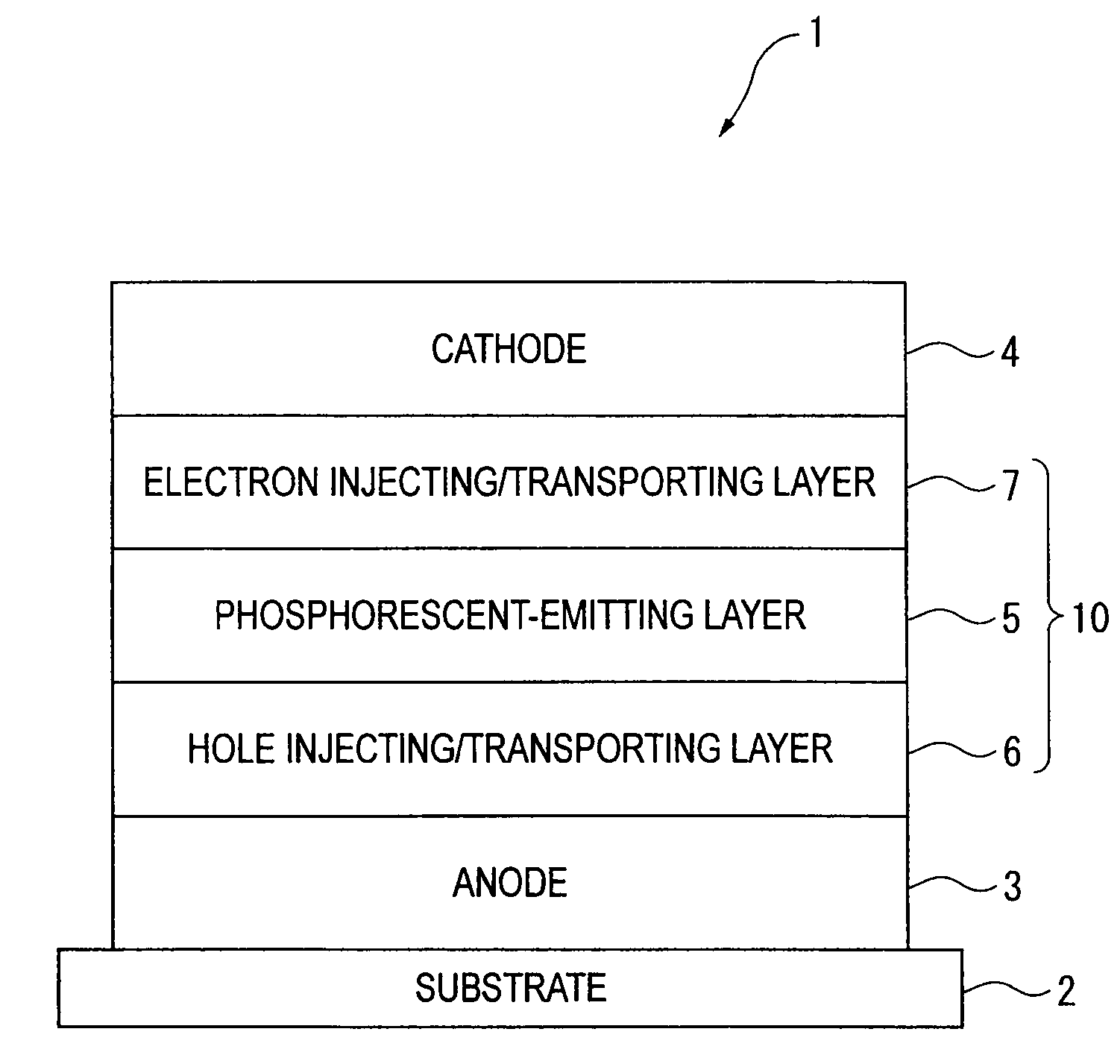
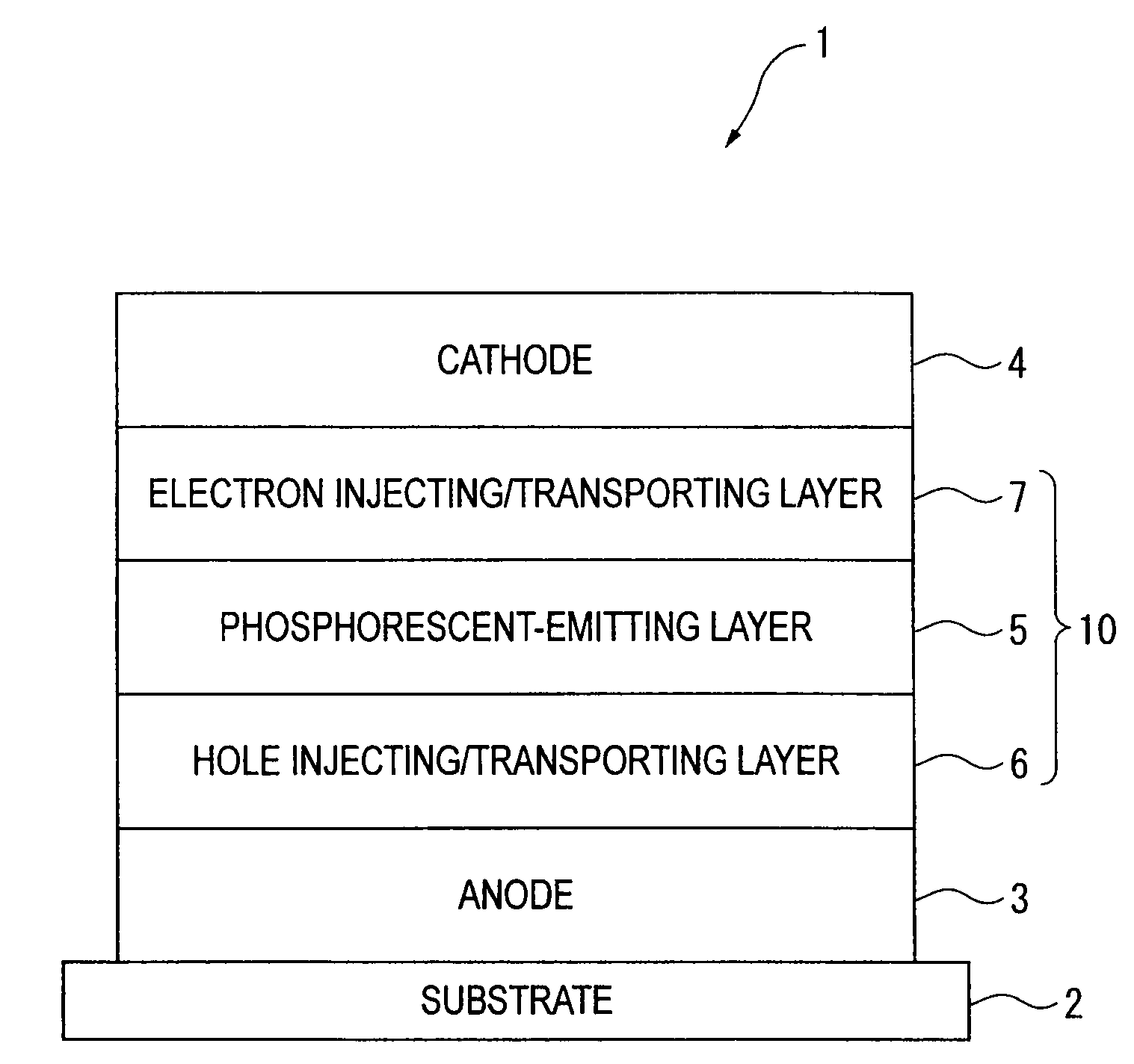
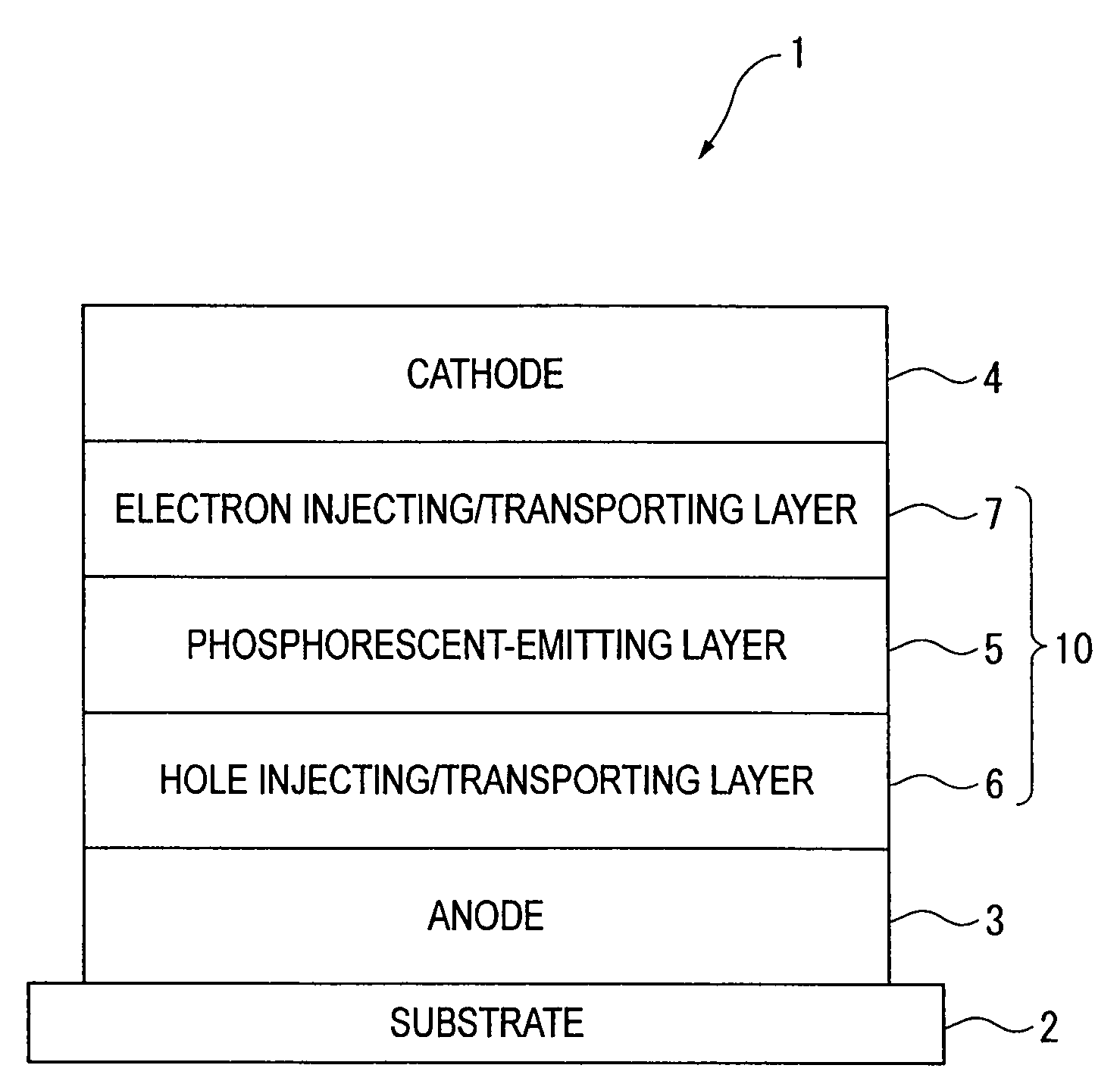
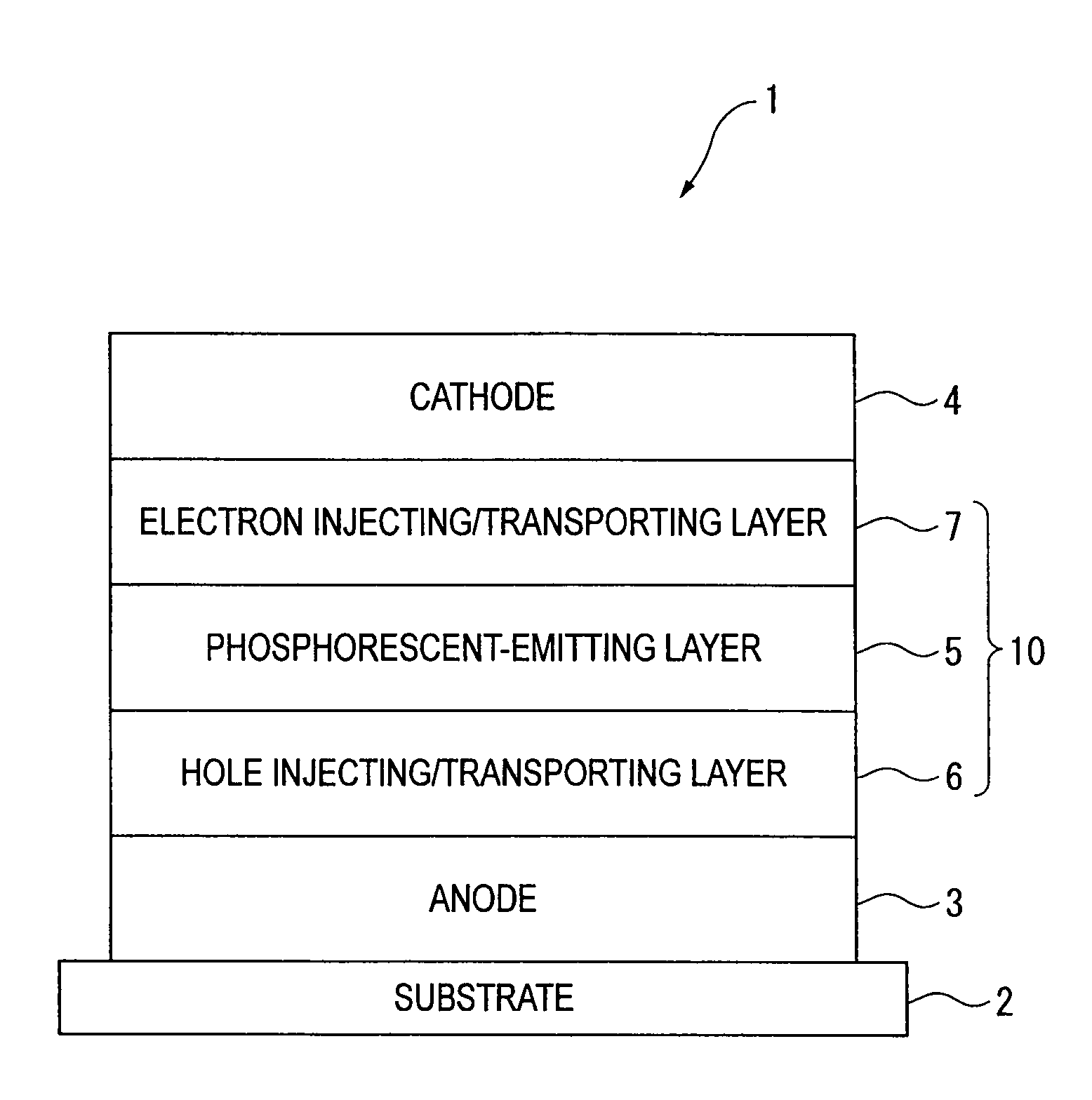
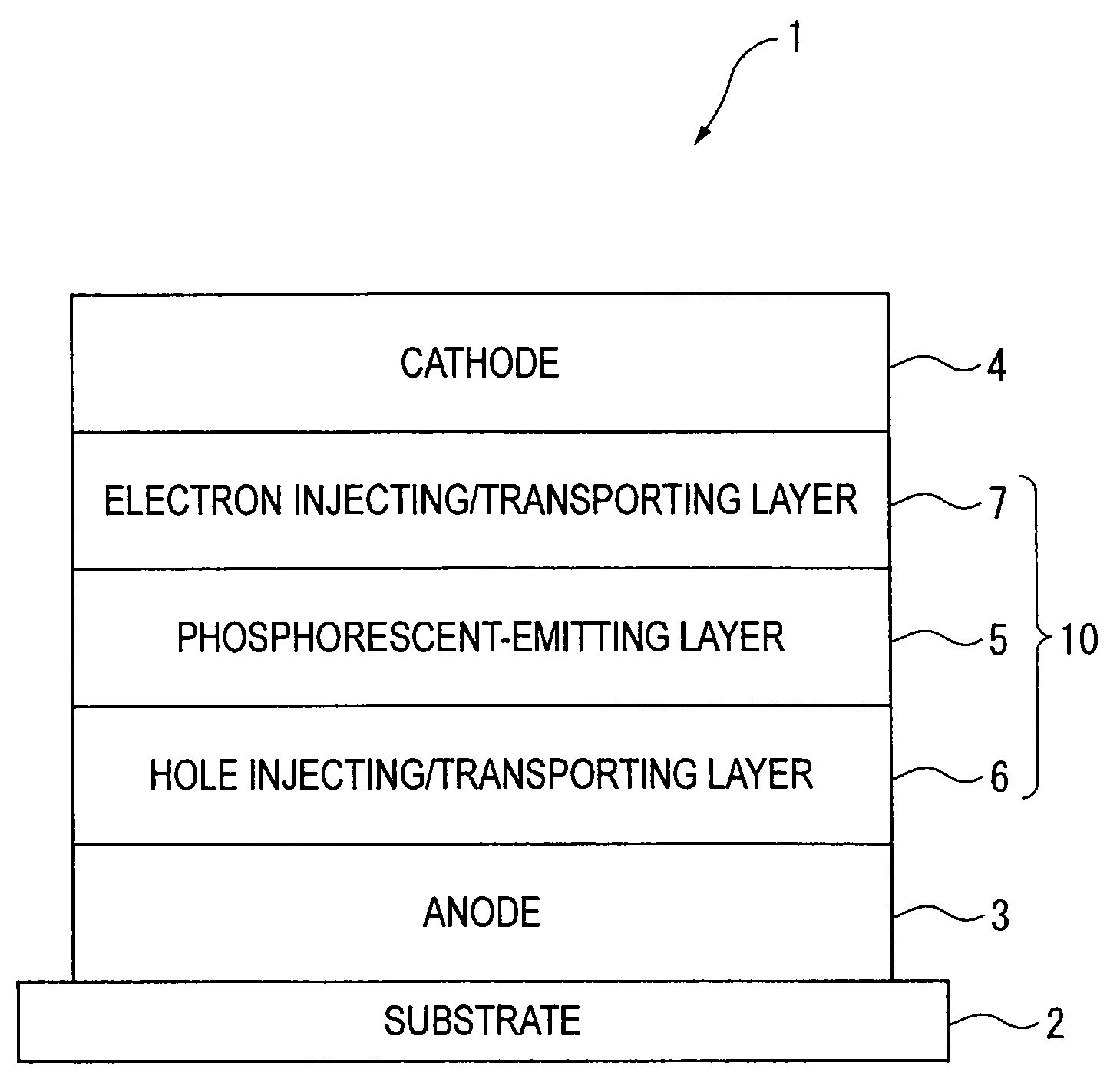
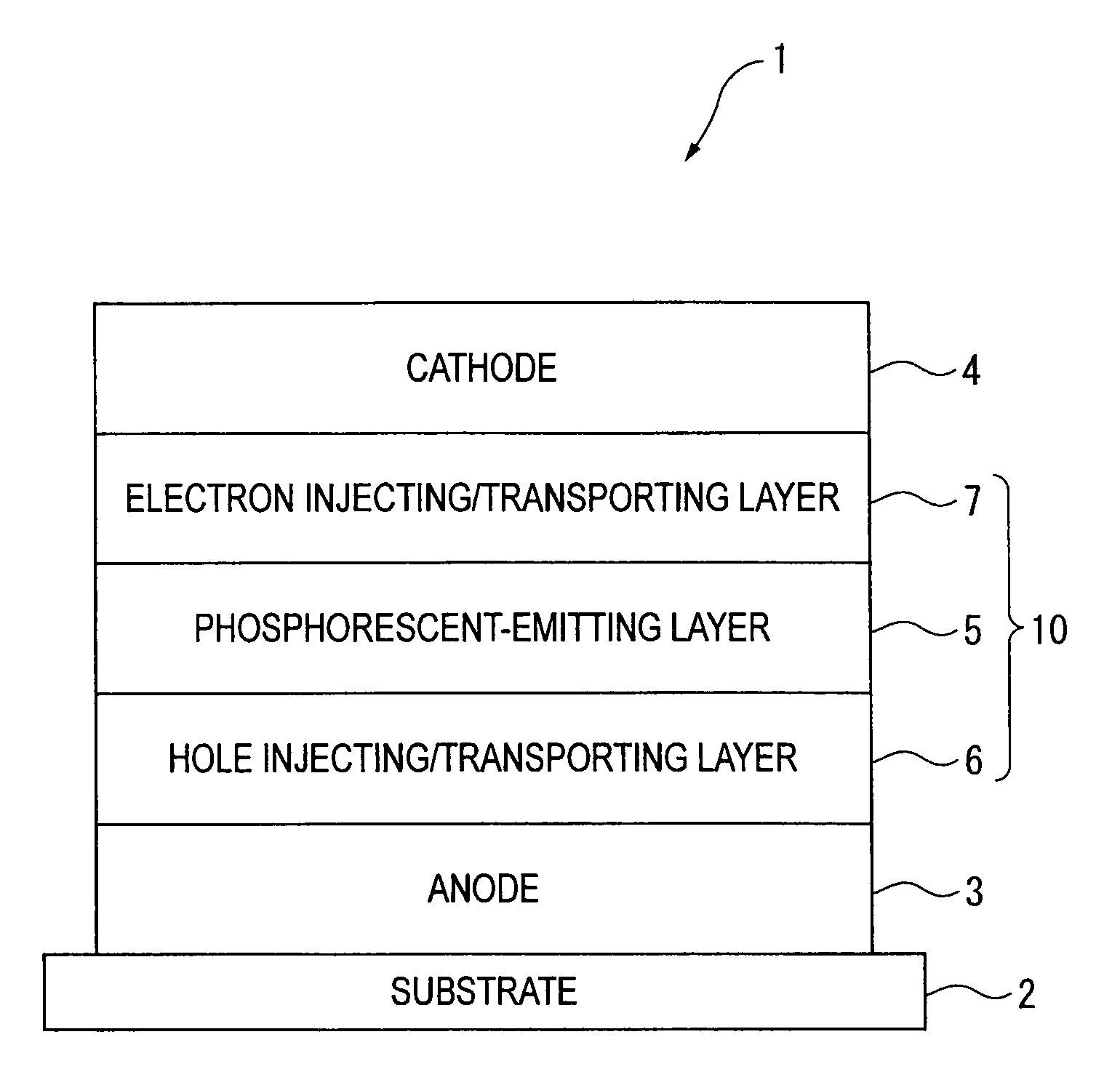
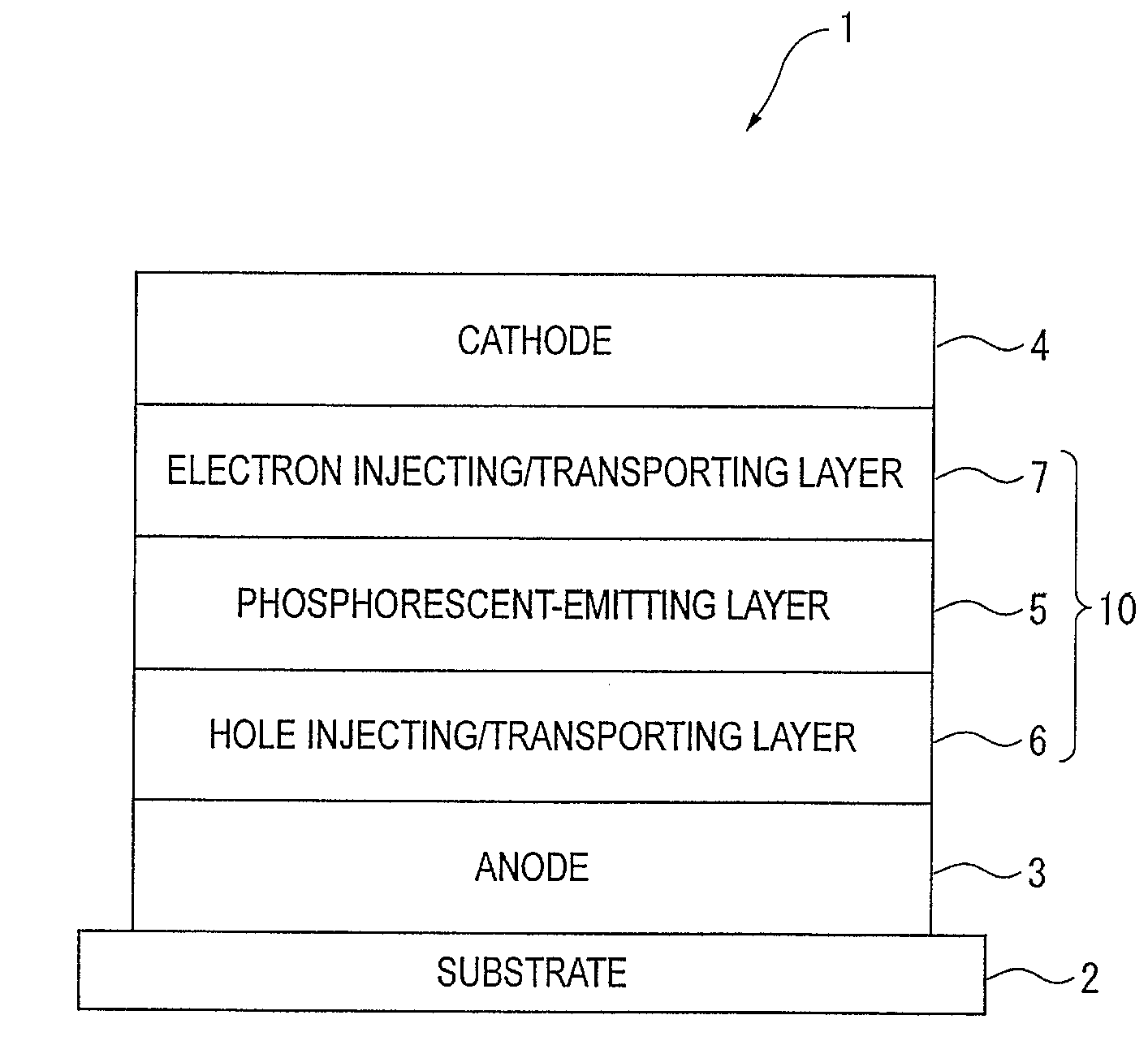
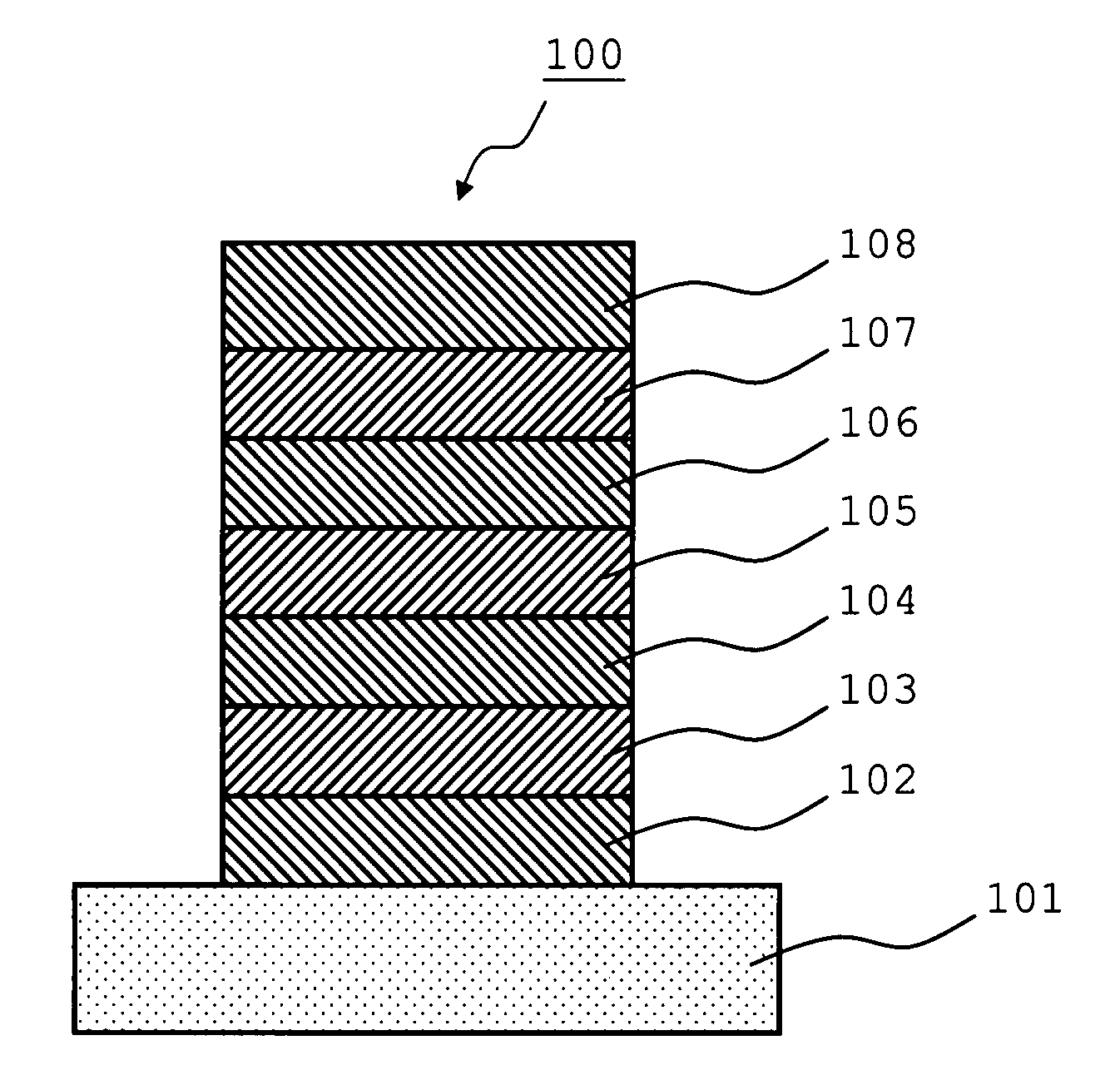
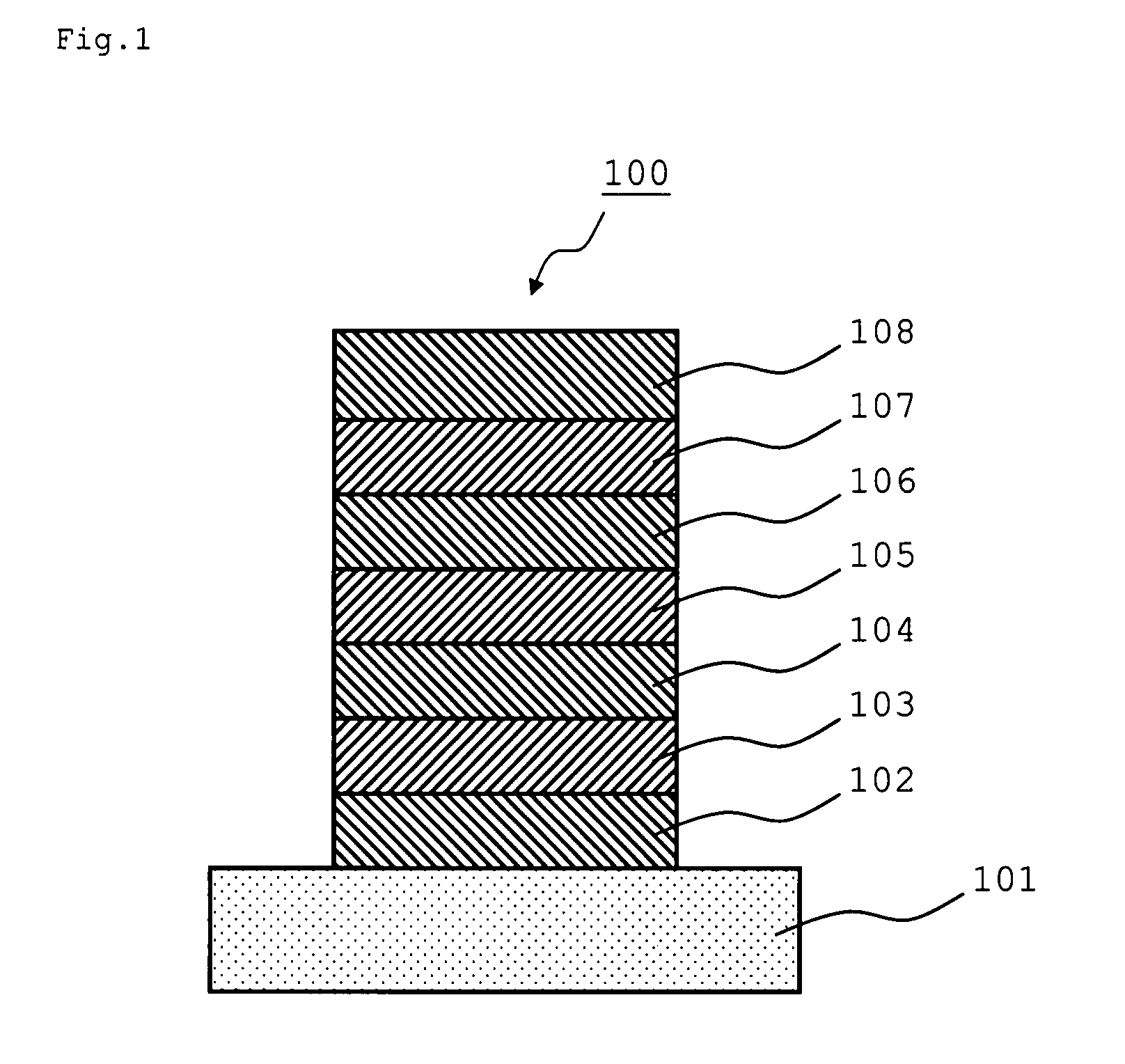
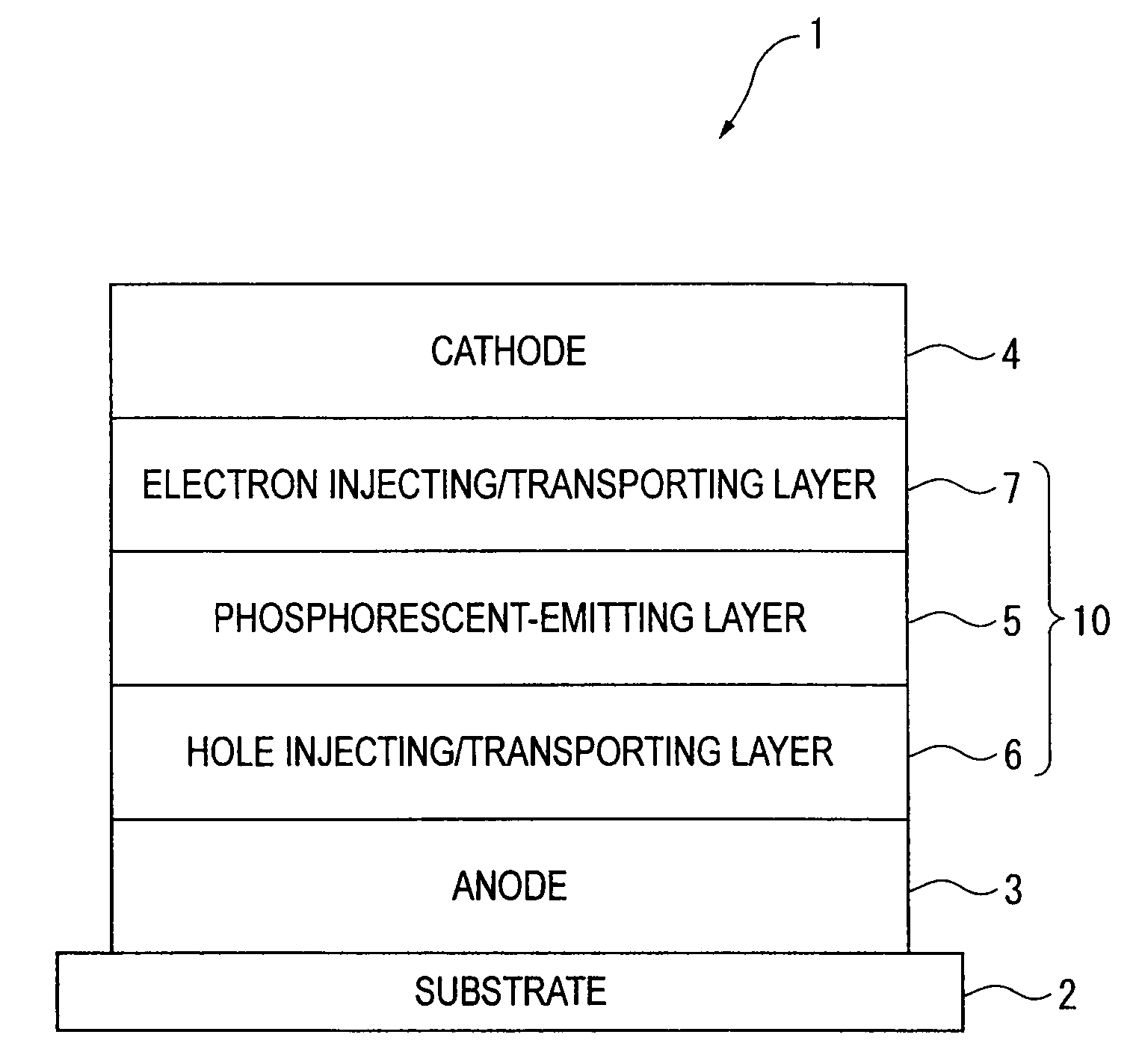
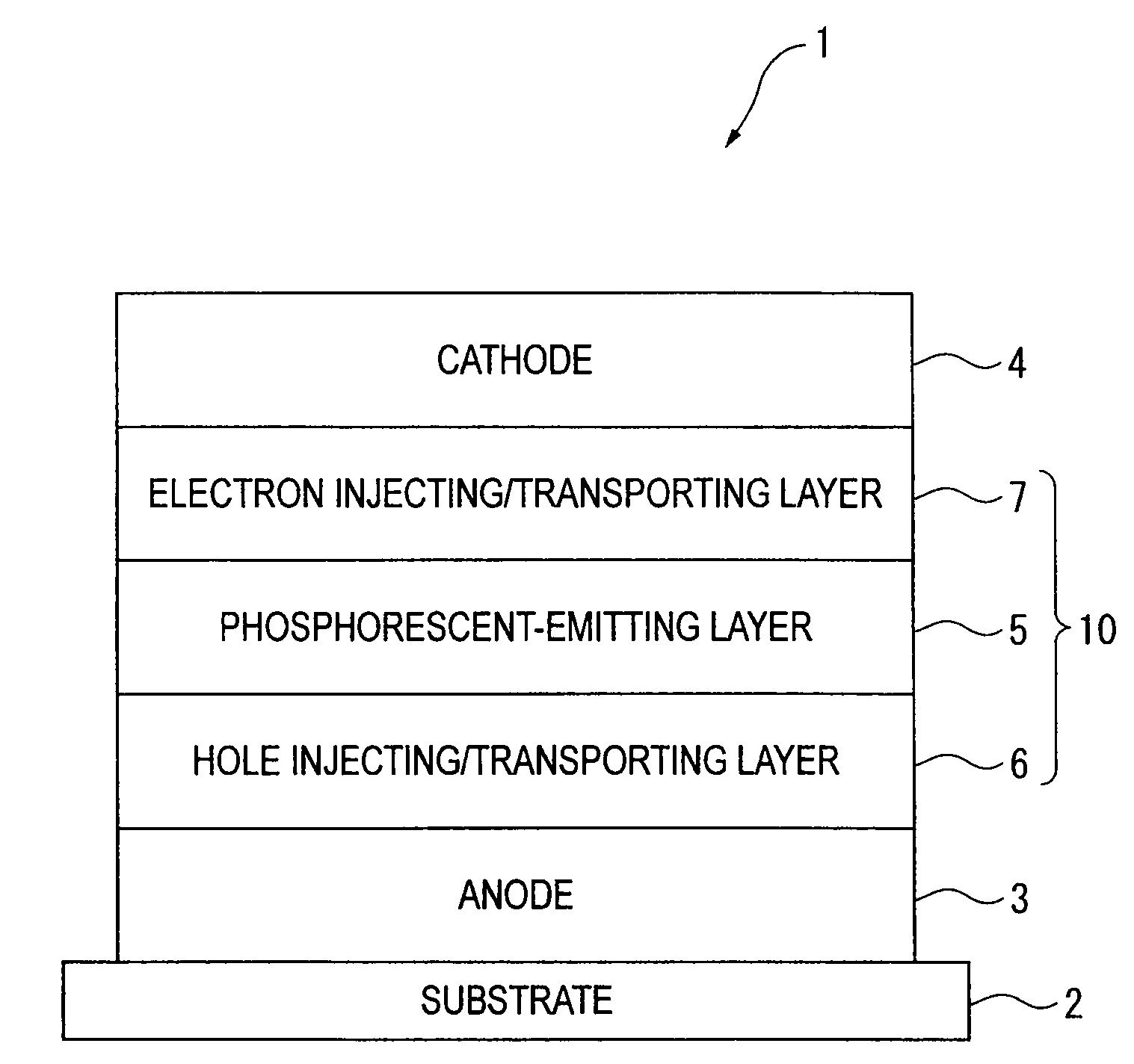
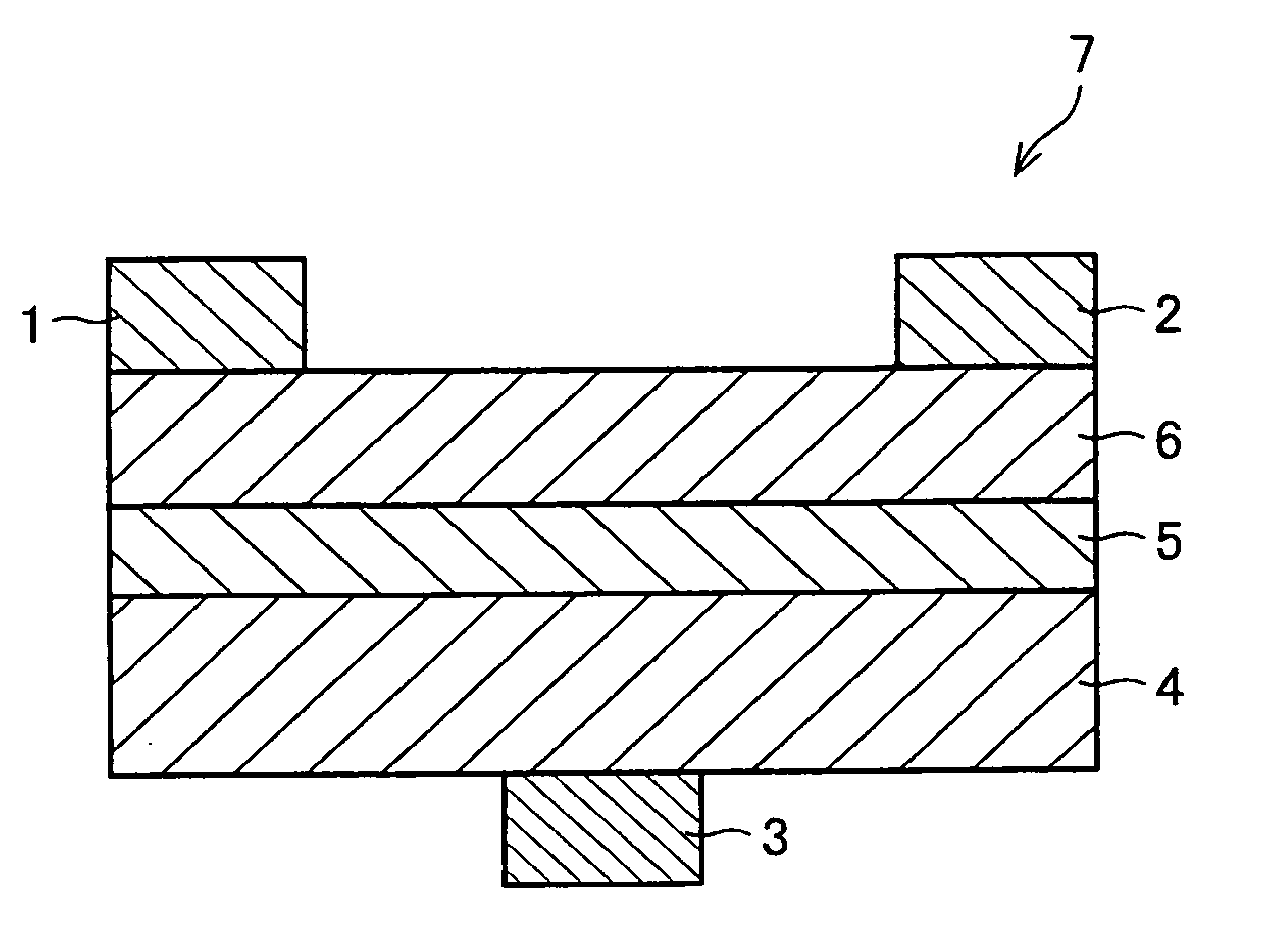
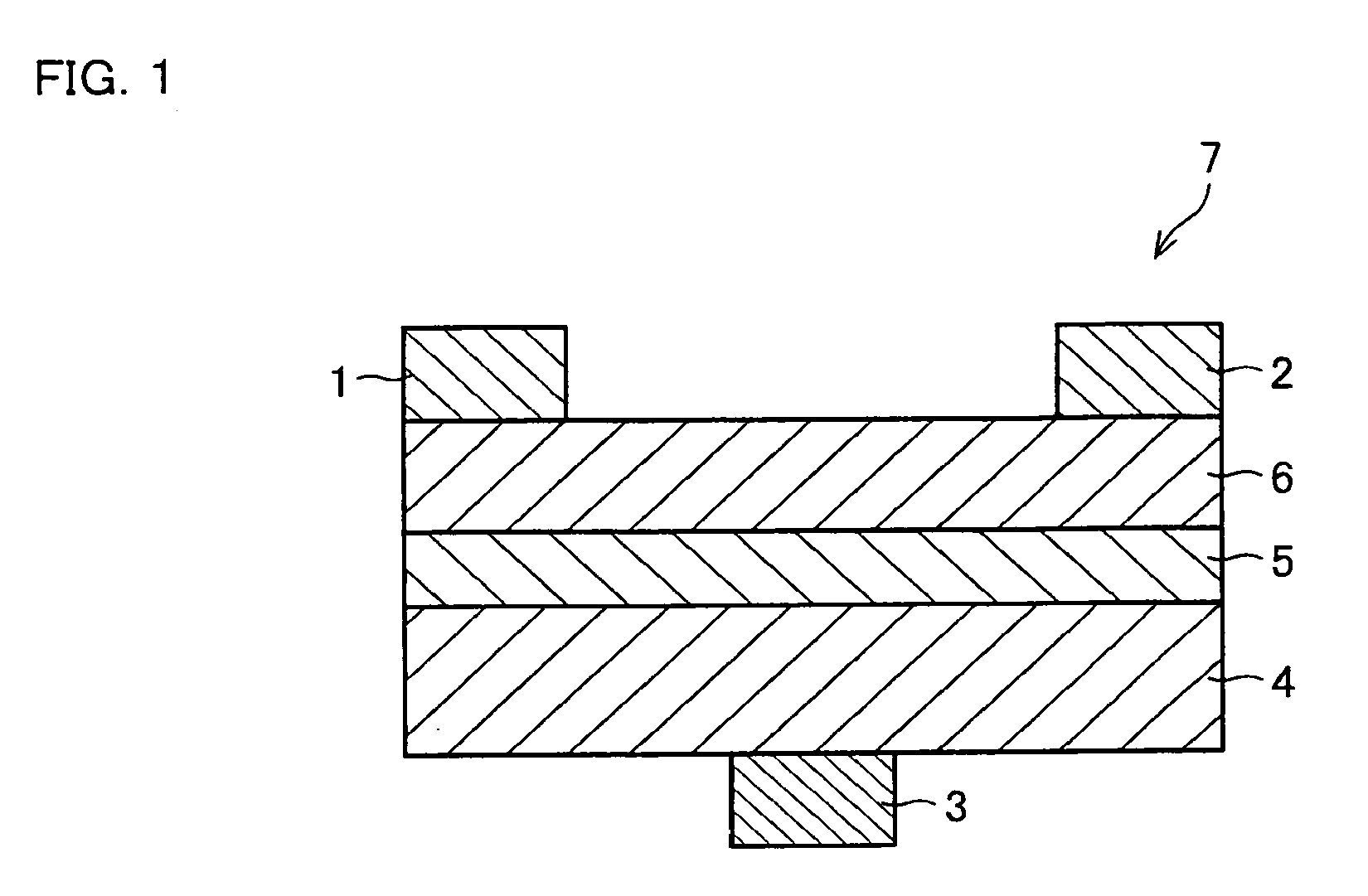
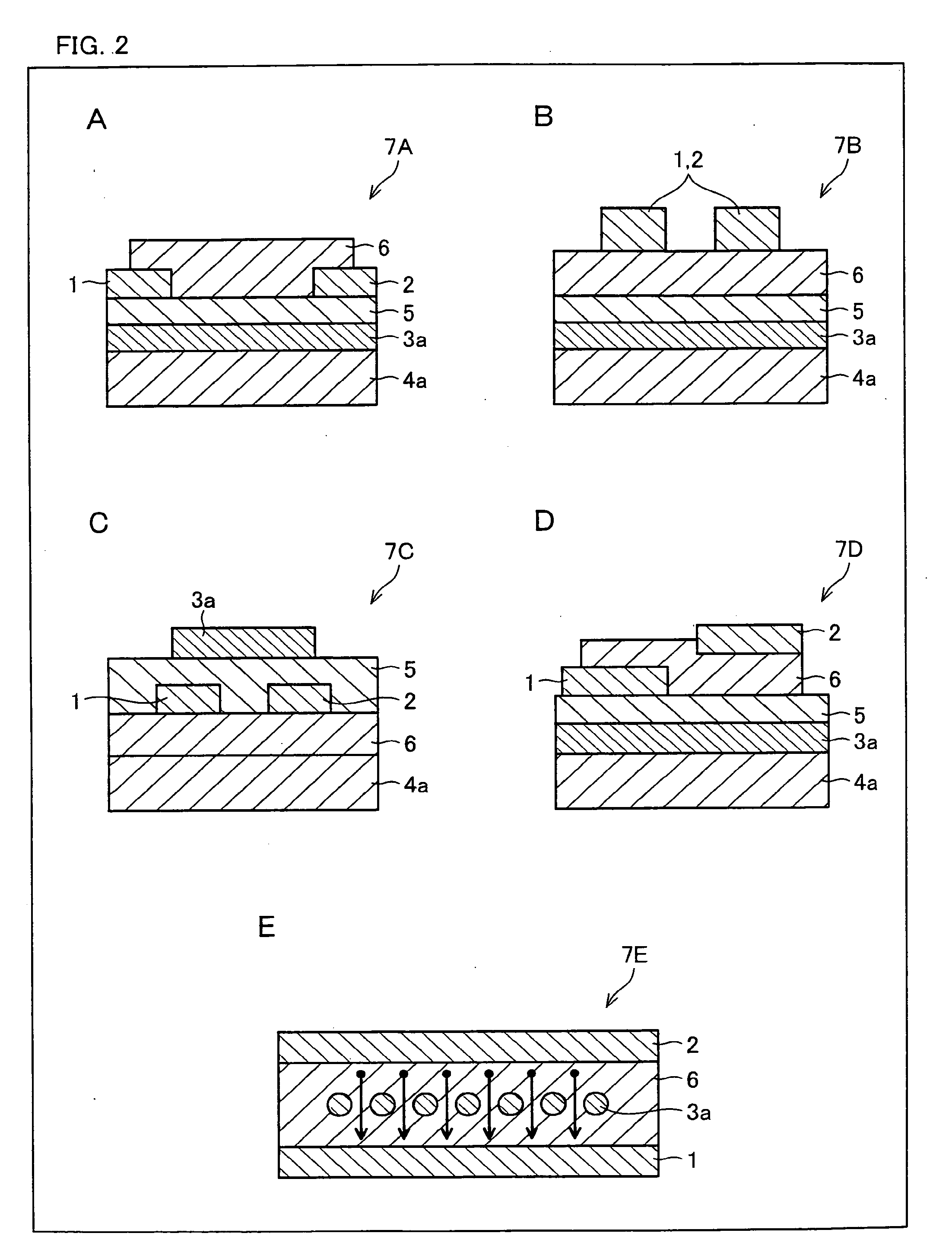
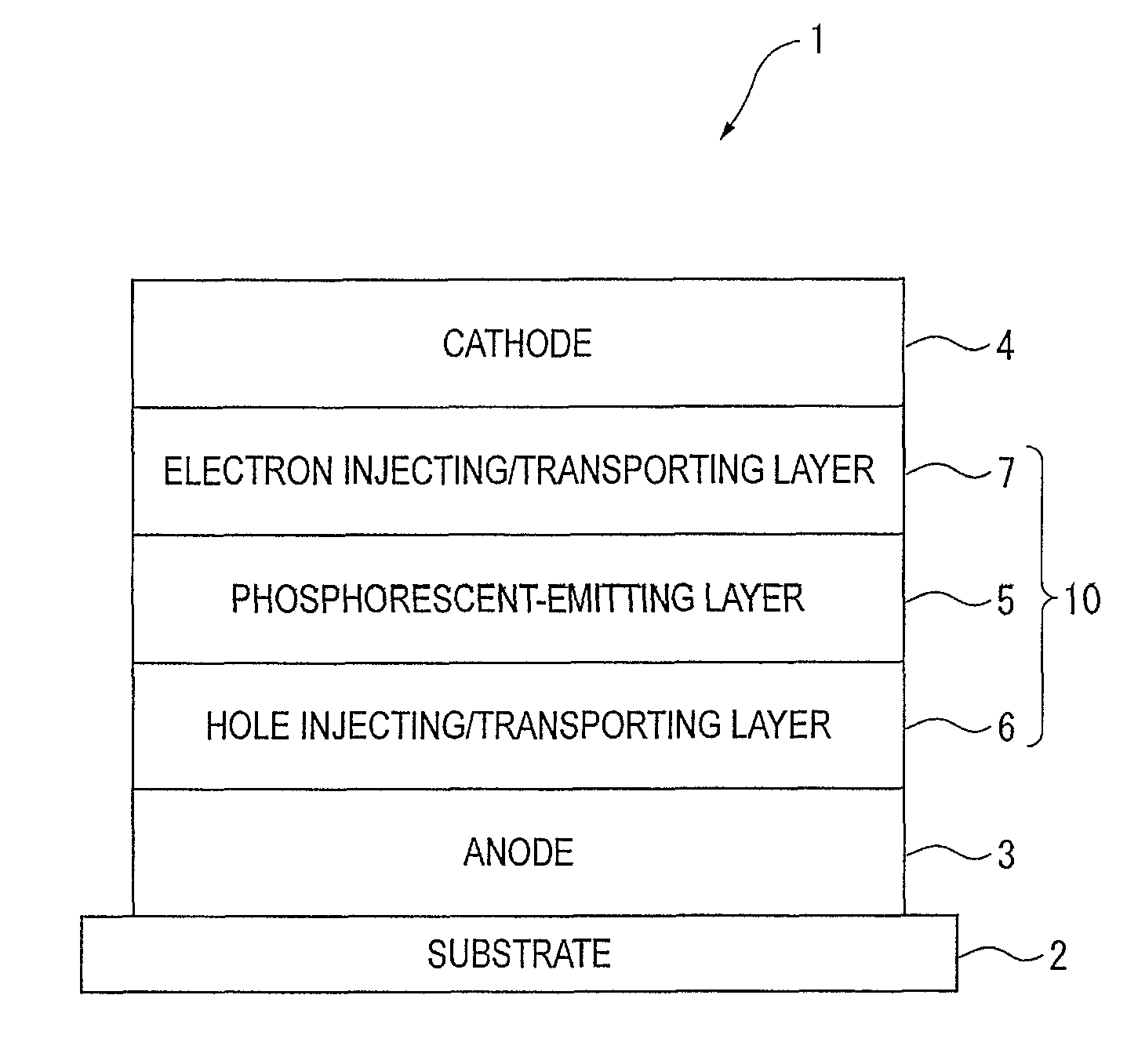
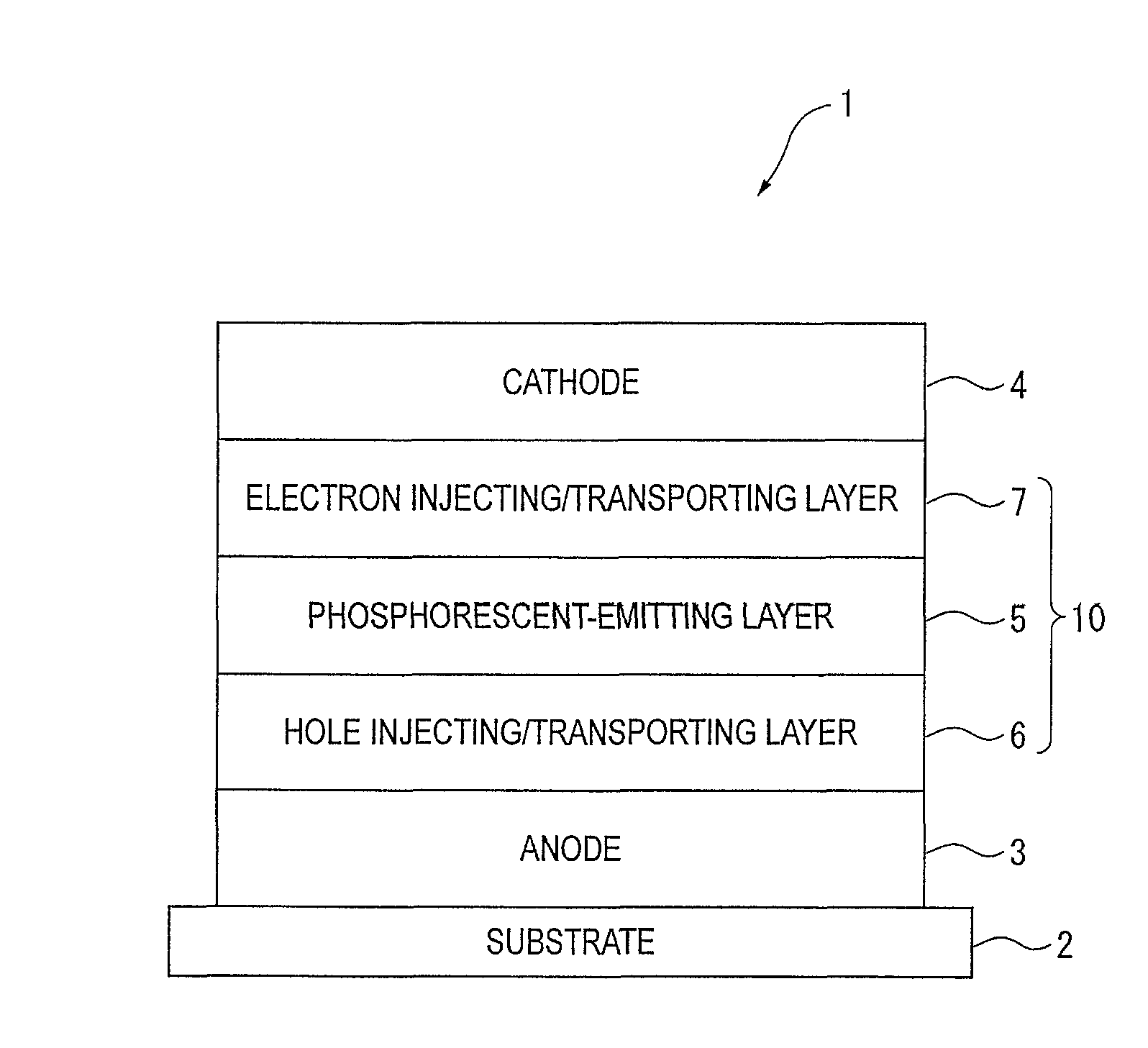
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. The organic thin-film layer includes at least one emitting layer. The at least one emitting layer contains at least one phosphorescent material and a host material represented by the following formula (1).In the formula, Ar1, Ar2, Ar3, B1, B2, B3 and B4 each represent a substituted or unsubstituted benzene ring or a substituted or unsubstituted condensed aromatic hydrocarbon ring selected from a naphthalene ring, a chrysene ring, a fluoranthene ring, a phenanthrene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a triphenylene ring, a benzo[a]triphenylene ring, a benzochrysene ring, a benzo[b]fluoranthene ring and a picene ring. p is 0 or 1.
Owner:IDEMITSU KOSAN CO LTD
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090045730A1Improve efficiencyLong life-timeOrganic chemistryDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
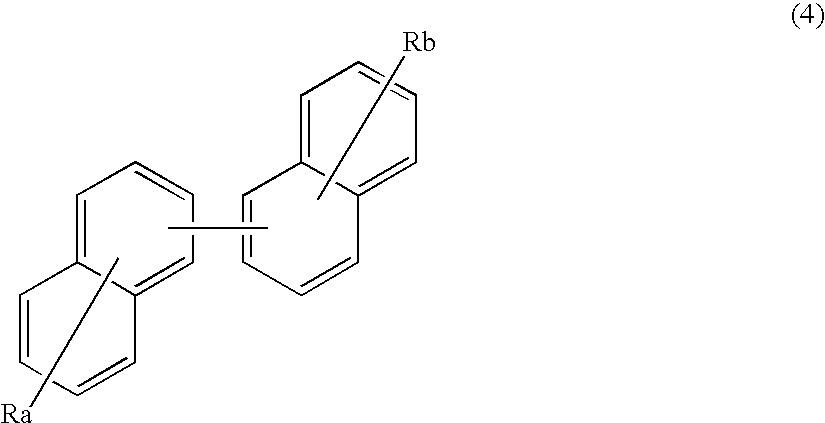
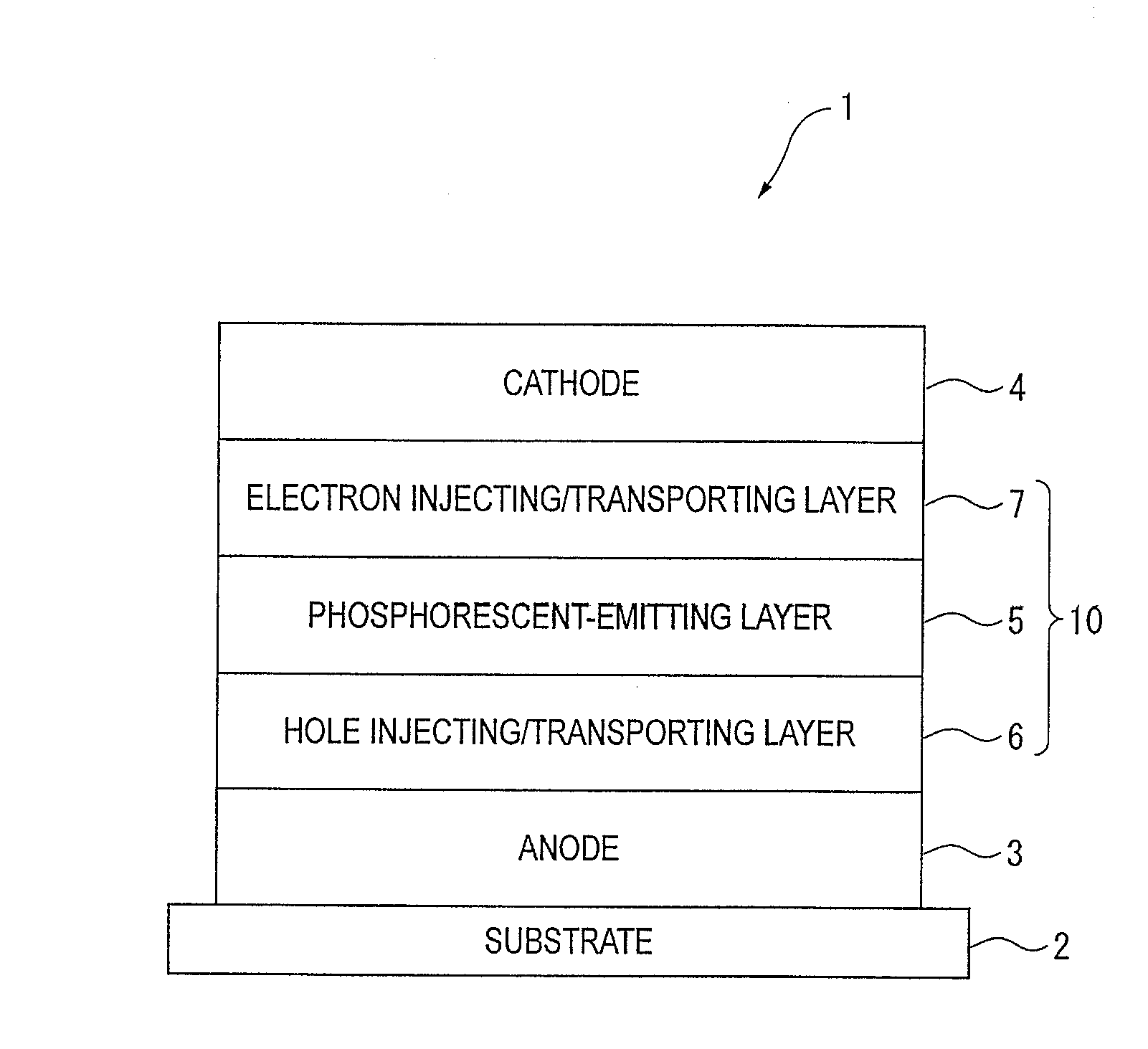
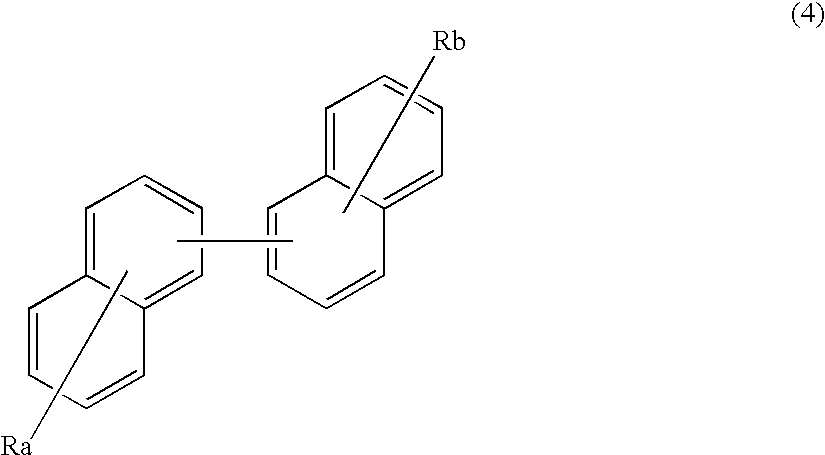
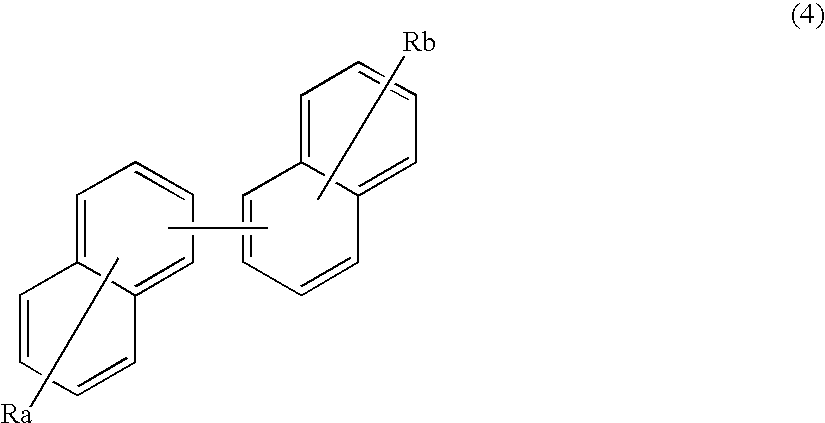
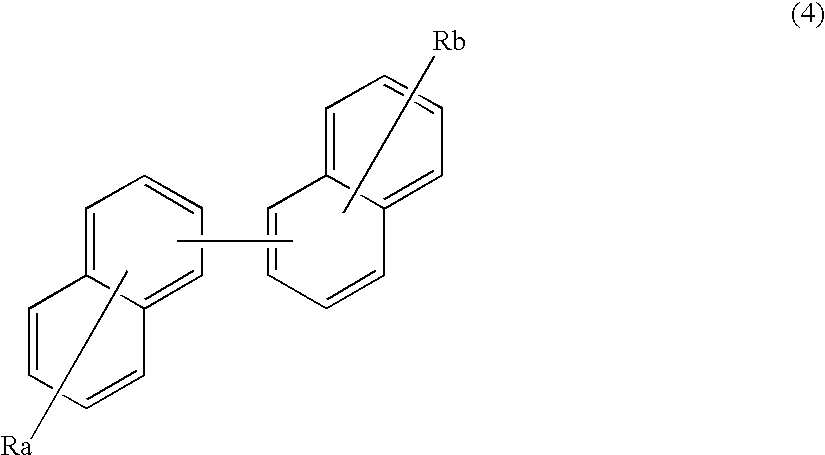
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. The organic thin-film layer includes at least one emitting layer. The at least one emitting layer contains at least one phosphorescent material and a host material represented by the following formula (1).Ra-Ar1-Rb (1)In the formula, Ar1, Ra and Rb each represent a substituted or unsubstituted benzene ring or a condensed aromatic hydrocarbon ring selected from a substituted or unsubstituted naphthalene ring, a substituted or unsubstituted chrysene ring, a substituted or unsubstituted fluoranthene ring, a substituted or unsubstituted phenanthrene ring, a substituted or unsubstituted benzophenanthrene ring, a substituted or unsubstituted dibenzophenanthrene ring, a substituted or unsubstituted triphenylene ring, a substituted or unsubstituted benzo[a]triphenylene ring, a substituted or unsubstituted benzochrysene ring, a substituted or unsubstituted benzo[b]fluoranthene ring and a substituted or unsubstituted picene ring. Substituents for Ra and Rb are not aryl groups.
Owner:IDEMITSU KOSAN CO LTD
Aryl- or heteroaryl-substituted benzene compounds
The present invention relates to aryl- or heteroaryl-substituted benzene compounds. The present invention also relates to pharmaceutical compositions containing these compounds and methods of treating cancer by administering these compounds and pharmaceutical compositions to subjects in need thereof. The present invention also relates to the use of such compounds for research or other non-therapeutic purposes.
Owner:EPIZYME
Organic electrolumescence device
InactiveUS20050038296A1High efficiency of light emissionSolution to short lifeOrganic chemistryMethine/polymethine dyesHydrogen atomOrganic group
Materials for organic electroluminescence devices are represented by following general formula [1]: general formula [1]wherein A represents a chrysene group X1 to X4 each independently represent a substituted or unsubstituted arylene group having 6 to 30 carbon atoms, X1 and X2 may be bonded to each other, X3 and X4 may be bonded to each other, Y1 to Y4 each independently represent an organic group represented by general formula [2], a to d each represent an integer of 0 to 2 and, a+b+c+d≧0; general formula [2] being: general formula [2]wherein R1 to R4 each independently represent hydrogen atom, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, a substituted or unsubstituted aryl group having 6 to 20 carbon atoms, cyano group or form a triple bond by a linkage of R1 and R2 or R3 and R4, Z represents a substituted or unsubstituted aryl group having 6 to 20 carbon atoms and n represents 0 or 1.
Owner:IDEMITSU KOSAN CO LTD
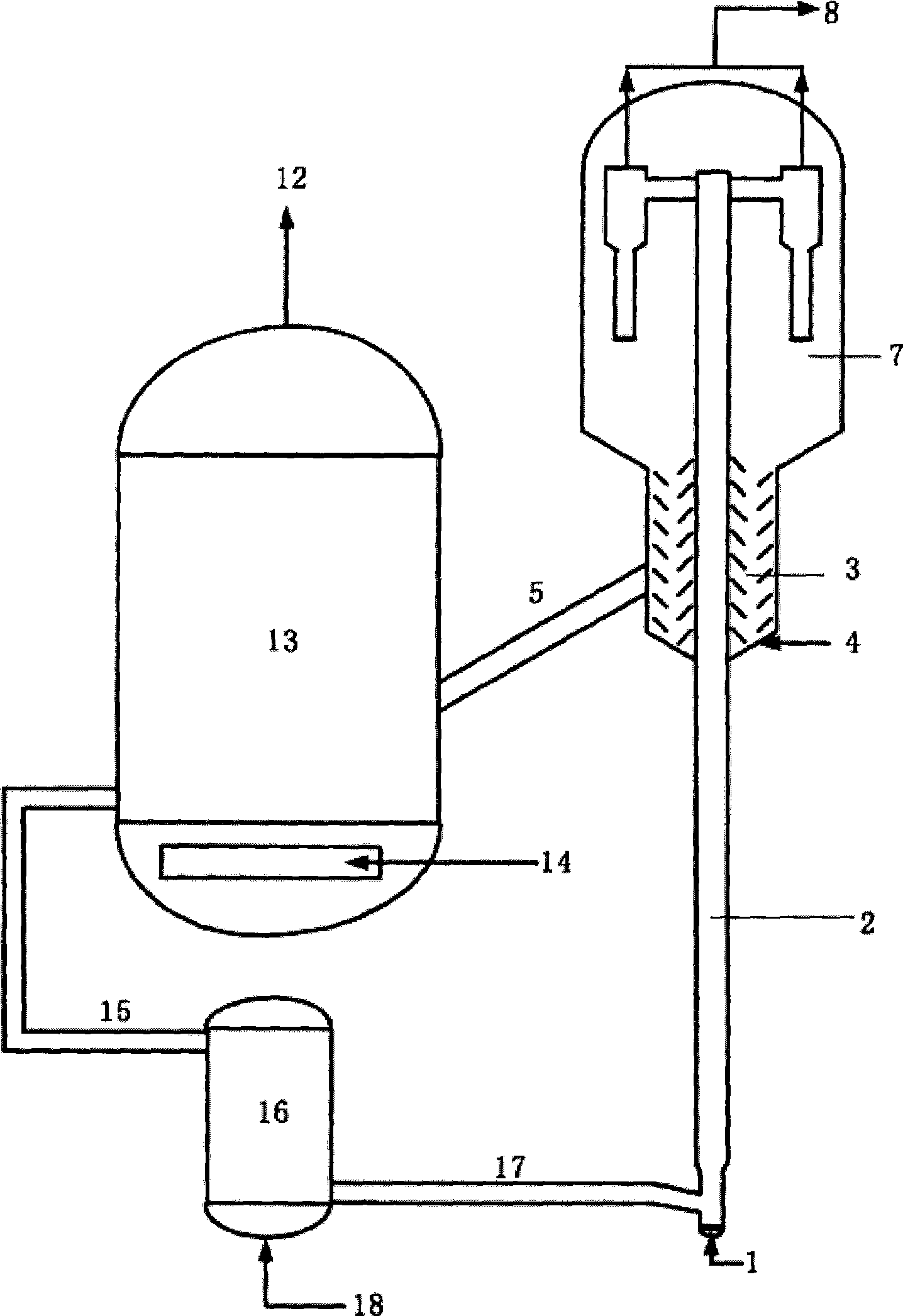
Sorbent for reducing sulfur content in hydrocarbon oils
ActiveCN101433821AHigh desulfurization activityEvenly distributedOther chemical processesHydrocarbon oils refiningSorbentRare earth
The invention relates to a sorbent for reducing sulfer content in hydrocarbon oil, which comprises 1 to 30 weight percent of rare earth faujasite, 5 to 40 weight percent of active metal oxide and 30 to 94 weight percent of carrier, wherein the carrier comprises alumina and zinc oxide. The mixture of the rare earth faujasite and the carrier is preformed into porous heat-resistant solid particles which are introduced with the metal active ingredient to prepare the sorbent; sulfer-containing light hydrocarbon oil raw material and hydrogen donors enter a reactor filled with the sorbent for separating reaction; materials and products after the separating reaction are sent to a subsequent separation system for product separation; sorbent to be regenerated after the reaction is burnt to be regenerated after steam stripping; and the regenerated sorbent is reduced by the hydrogen donors and returned to the reactor for recycling. The sorbent realizes deep removal of sulfer in the light hydrocarbon oil; meanwhile, the product gasoline has high octane number, low benzene content and high strength.
Owner:CHINA PETROLEUM & CHEM CORP +1
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090009067A1Improve efficiencyLong life-timeDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmBenzo(c)phenanthrene
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. In the organic electroluminescence device, the organic thin-film layer includes at least one emitting layer, and the at least one emitting layer contains: at least one phosphorescent material; and a host material represented by the following formula (1).Ra—Ar1—Ar2—Rb (1)In the formula, Ar1, Ar2, Ra and Rb each represent a substituted or unsubstituted benzene ring or a substituted or unsubstituted condensed aromatic hydrocarbon group selected from a group consisting of a naphthalene ring, a chrysene ring, a fluoranthene ring, a triphenylene ring, a phenanthrene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a benzotriphenylene ring, a benzochrysene ring, a picene ring and a benzo[b]fluoranthene ring.
Owner:IDEMITSU KOSAN CO LTD
Benzofluorene compound, emission materials and organic electroluminescent device
InactiveUS20080160347A1Improve featuresEasy to synthesizeOrganic chemistryDischarge tube luminescnet screensArylOrganic electroluminescence
Provided is a benzofluorene compound which exhibits excellent performances when applied to an organic electroluminescent device.In the benzofluorene compound, a central five-membered ring in a benzofluorene skeleton is substituted with aryl, and a benzene ring condensed to the five-membered ring is substituted with aryl, diarylamino and the like.
Owner:JNC CORP
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS8330350B2Improve efficiencyLong lastingDischarge tube luminescnet screensElectroluminescent light sourcesFluorantheneHost material
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. In the organic electroluminescence device, the organic thin-film layer includes at least one emitting layer, and the at least one emitting layer includes at least one phosphorescent material and a host material represented by the following Formula (1).Ra—Ar1—Ar2—Rb (1)In Formula (1):Ra and Rb each represent a substituted or non-substituted benzene ring or a substituted or non-substituted condensed aromatic hydrocarbon ring selected from a group consisting of a naphthalene ring, a chrysene ring, a fluoranthene ring, a triphenylene ring, a phenanthrene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a benzotriphenylene ring, a benzochrysene ring and a picene ring; andAr1 and Ar2 each represent a substituted or non-substituted benzene ring or a substituted or non-substituted condensed aromatic hydrocarbon ring selected from a group consisting of a naphthalene ring, a chrysene ring, a fluoranthene ring, a triphenylene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a benzotriphenylene ring, a benzochrysene ring and a picene ring.
Owner:IDEMITSU KOSAN CO LTD
Novel fused polycyclic aromatic compound, process for producing the same, and use thereof
ActiveUS20100065826A1Improve accuracyEnhanced intermolecular interactionsSolid-state devicesSemiconductor/solid-state device manufacturingSolubilityPolycyclic compound
In one embodiment of the present invention, a novel fused polycyclic aromatic compound of the present invention is (a) a compound including a benzodichalcogenophenobenzodichalcogenophene (BXBX) skeleton further having an aromatic ring(s) located outside the BXBX skeleton, or (b) a compound including a BXBX skeleton in which a benzene ring is substituted with a heterocyclic ring. The compound can strengthen intermolecular interaction due to greater π electron orbits. This improves an electron field effect mobility of an organic semiconductor device that is manufactured by use of the compound as an organic semiconductor material. Further, since the number of fused rings included in the compound is small, the compound does not cause problems that generally occur in compounds having an extremely large number of fused rings, i.e., poor solubility in solvent and poor atmospheric stability due to high affinity to oxygen. As a result, the fused polycyclic aromatic compound of the present invention can be preferably used as an organic semiconductor material.
Owner:NIPPON KAYAKU CO LTD
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS8154195B2Improve efficiencyLong lastingDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmBenzene
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. In the organic electroluminescence device, the organic thin-film layer includes at least one emitting layer, and the at least one emitting layer contains: at least one phosphorescent material; and a host material represented by the following formula (1).Ra—Ar1—Ar2—Rb (1)In the formula, Ar1, Ar2, Ra and Rb each represent a substituted or unsubstituted benzene ring or a substituted or unsubstituted condensed aromatic hydrocarbon group selected from a group consisting of a naphthalene ring, a chrysene ring, a fluoranthene ring, a triphenylene ring, a phenanthrene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a benzotriphenylene ring, a benzochrysene ring, a picene ring and a benzo[b]fluoranthene ring.
Owner:IDEMITSU KOSAN CO LTD
Organic electroluminescent material and application thereof
The invention relates to a 1,2-benzo[a]anthracene derivative and an organic electroluminescent device containing the compound. The general structural formula of the compound is shown in a structural general formula (1), wherein A1 and A2 are independently selected from aromatic groups with 6 to 25 nuclear carbon atoms respectively, or selected from substituent groups represented by the general formulae (1) to (4). The material of the invention can be preferably used as a luminous body, and simultaneously can be used as a transmission material. The electroluminescent device manufactured by the material of the invention has the high performances of low drive voltage, high luminescent efficiency and the like.
Owner:TSINGHUA UNIV +2
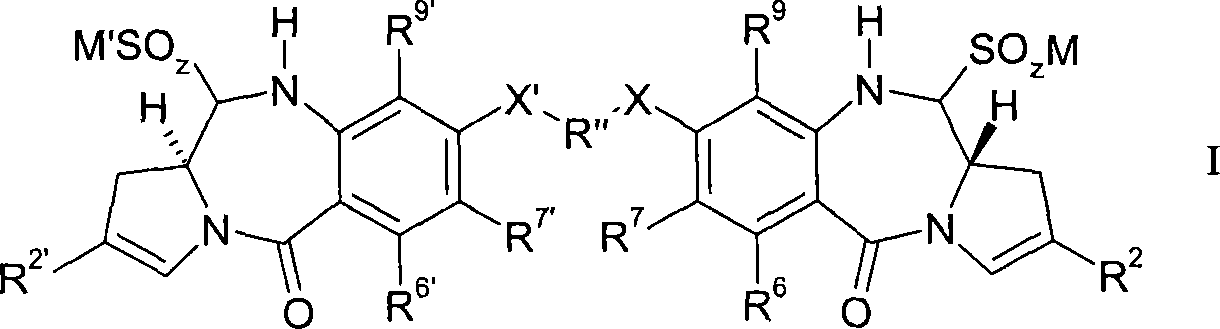
Pyrrolobenzodiazepines compound
Compounds of the formula: (I) or solvate thereof, wherein: R<2> is an optionally substituted C5-20 aryl group; R<6> and R<9> are independently selected from H, R, OH, OR, SH, SR, NH2, NHR, NRR', nitro, Me3Sn and halo; where R and R' are independently selected from optionally substituted C1-12 alkyl, C3-20 heterocyclyl and C5-20 aryl groups; R<7> is selected from H, R, OH, OR, SH, SR, NH2, NHR, NHRR', nitro, Me3Sn and halo; R'' is a C3-12 alkylene group, which chain may be interrupted by one or more heteroatoms and / or aromatic rings; X is selected from O, S, or NH; z is 2 or 3; M is a monovalent pharmaceutically acceptable cation; R<2>', R<6>', R<7>', R<9>', X' and M' are selected from the same groups as R<2>, R<6>, R<7>, R<9>, X and M respectively, or M and M' may together represent a divalent pharmaceutically acceptable cation.
Owner:MEDIMMUNE LTD
Corrosion-inhibition drag reducer for alkyl porphyrin compound natural gas pipelines and preparation method of corrosion-inhibition drag reducer
ActiveCN102838606AImprove anti-corrosion performanceOrganic chemistryPipeline systemsBenzaldehydePorphyrin
The invention relates to a corrosion-inhibition drag reducer for alkyl porphyrin compound natural gas pipelines of and a preparation method of the corrosion-inhibition drag reducer. The preparation method comprises the steps as follows: firstly, benzaldehyde, 4-hydroxyl benzaldehyde and pyrrole are used as raw materials to be reacted so as to prepare the mixture of six kinds of porphyrin compounds (H); then, the mixture H of the porphyrin compounds is reacted with acrylic acid long-chain alkyl esters to prepare six kinds of alkyl orphyrin compounds, wherein each kind of the compounds is 15 to 20wt%; and the prepared compounds are dissolved in 30 to 50wt% of solvent prepared with p-dioxane so as to form the corrosion-inhibition type drag reducer for the alkyl porphyrin compound natural gas pipelines. The proportion is that the mole ratio of methanal to the 4-hydroxyl benzaldehyde is (0.8:1) to (1.2:1); the mole ratio of the benzaldehyde to the pyrrole is (0.8:2) to (1.2:2); and the mole ratio of the acrylic acid long-chain alkyl esters to the pyrrole synthetizing H is (1:1) to (2:1). The drag reducer provided by the invention has the advantages of good drag reduction and anticorrosion property.
Owner:PIPECHINA SOUTH CHINA CO
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS8779655B2Improve efficiencyLong lastingDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmBenzo(c)phenanthrene
Owner:IDEMITSU KOSAN CO LTD
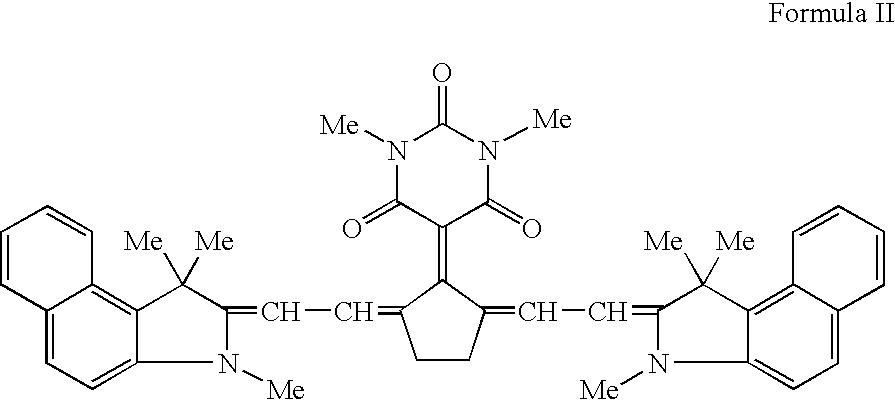
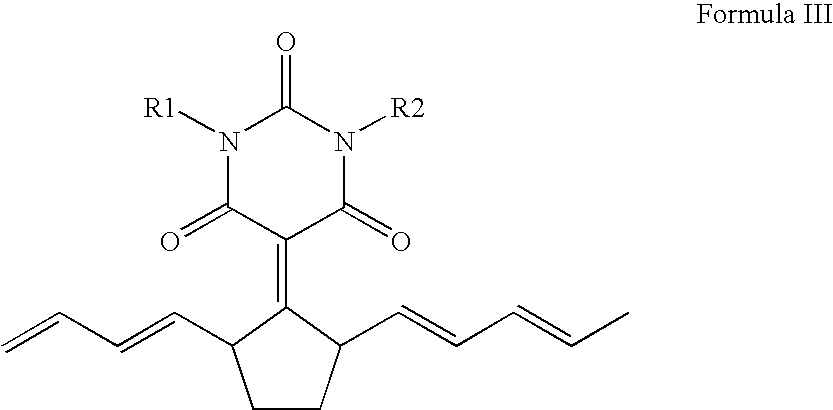
Stabilizers and anti-fade agents for use in infrared sensitive leuco dye compositions
InactiveUS20050053863A1Rapidly developable and light stable color forming compositionComposition is stablePhotosensitive materialsPhotomechanical apparatusColor imageLeuco dye
Compositions and methods for production of color images having increased light stability and reduced browning are described. The color forming composition can include a leuco dye, an infrared absorber, and at least one of a stabilizer and an anti-fade agent. The color forming compositions can be stabilized such that less than about a 30% decrease in optical density occurs over a three year period. The stabilizers can include chroman, thiolane-nickel complexes, spiroindanes, while suitable anti-fade agents can include vitamin E, vitamin E analogs, astaxanthin, chroman, ascorbic acid, carotene, and mixtures thereof. The color forming compositions are ambient light stable and are useful in forming images on a wide variety of substrates such as optical disks.
Owner:HEWLETT PACKARD DEV CO LP
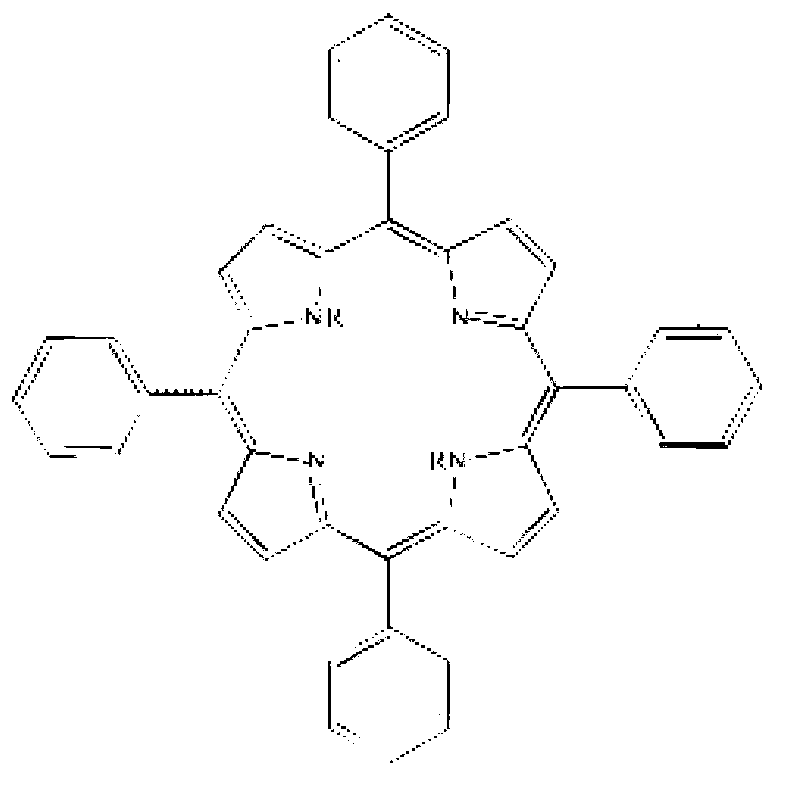
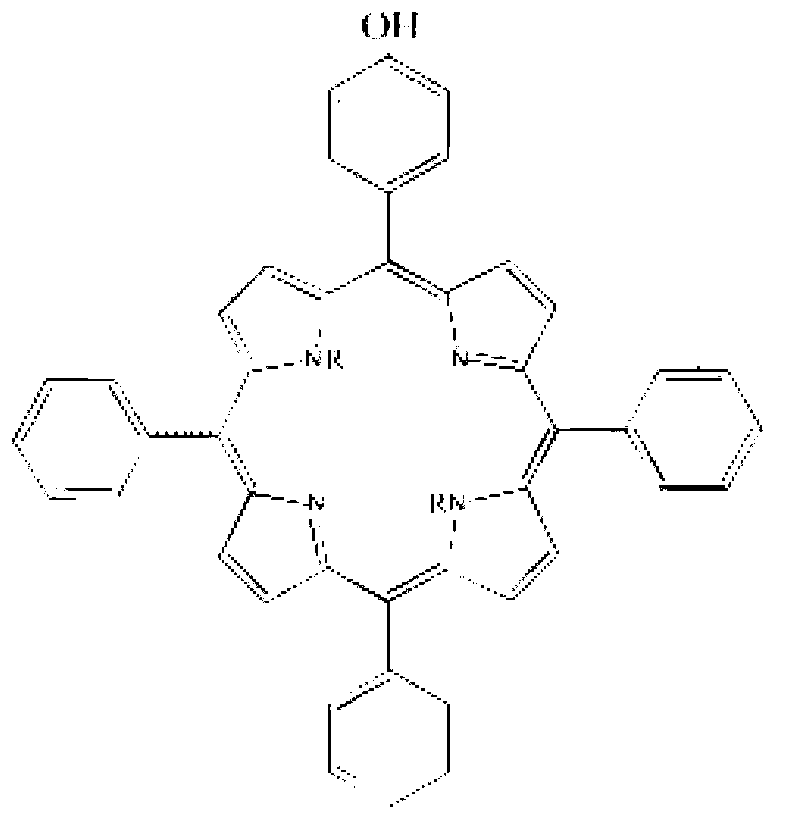
2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds and organic light emitting device containing same
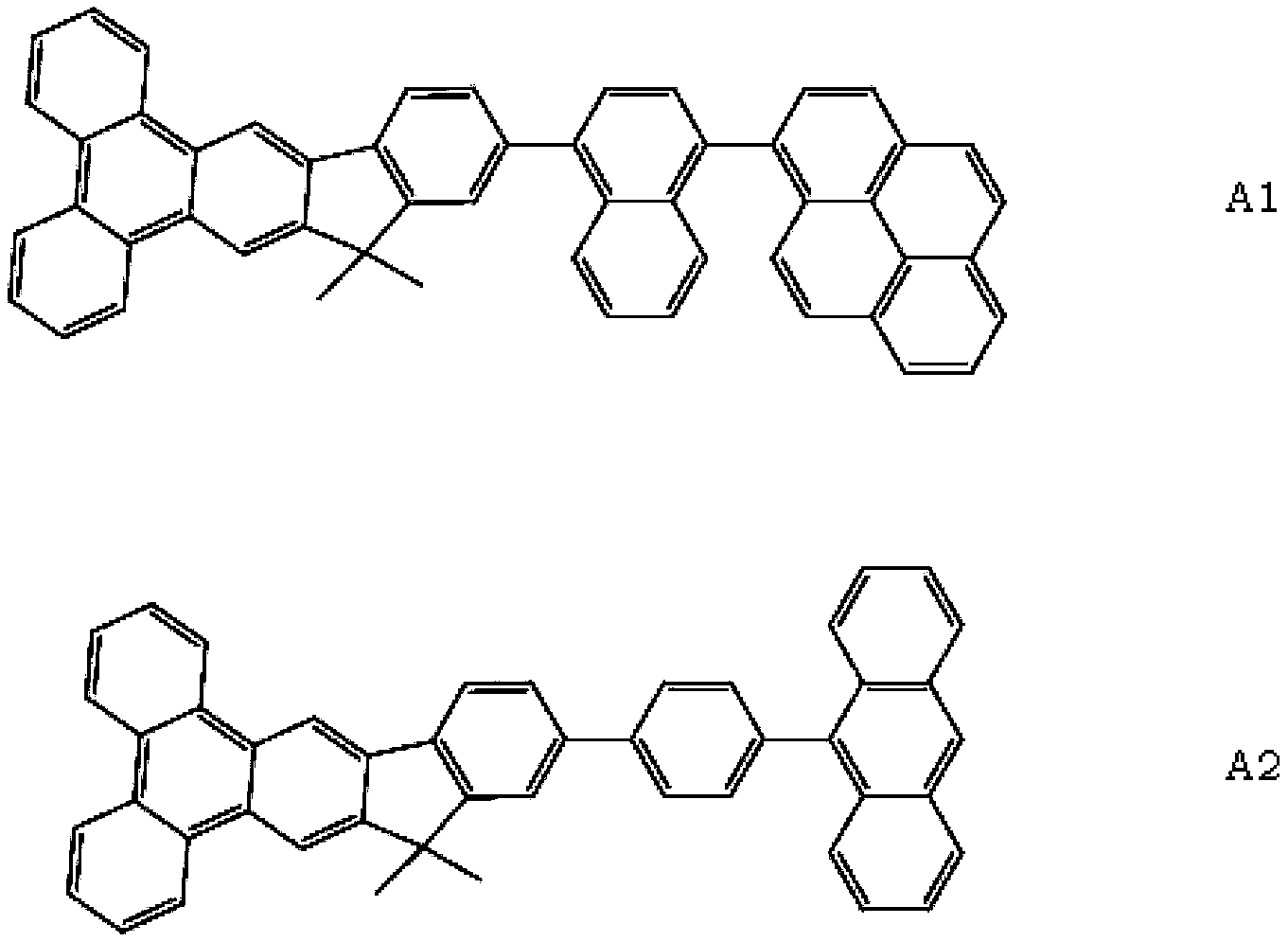
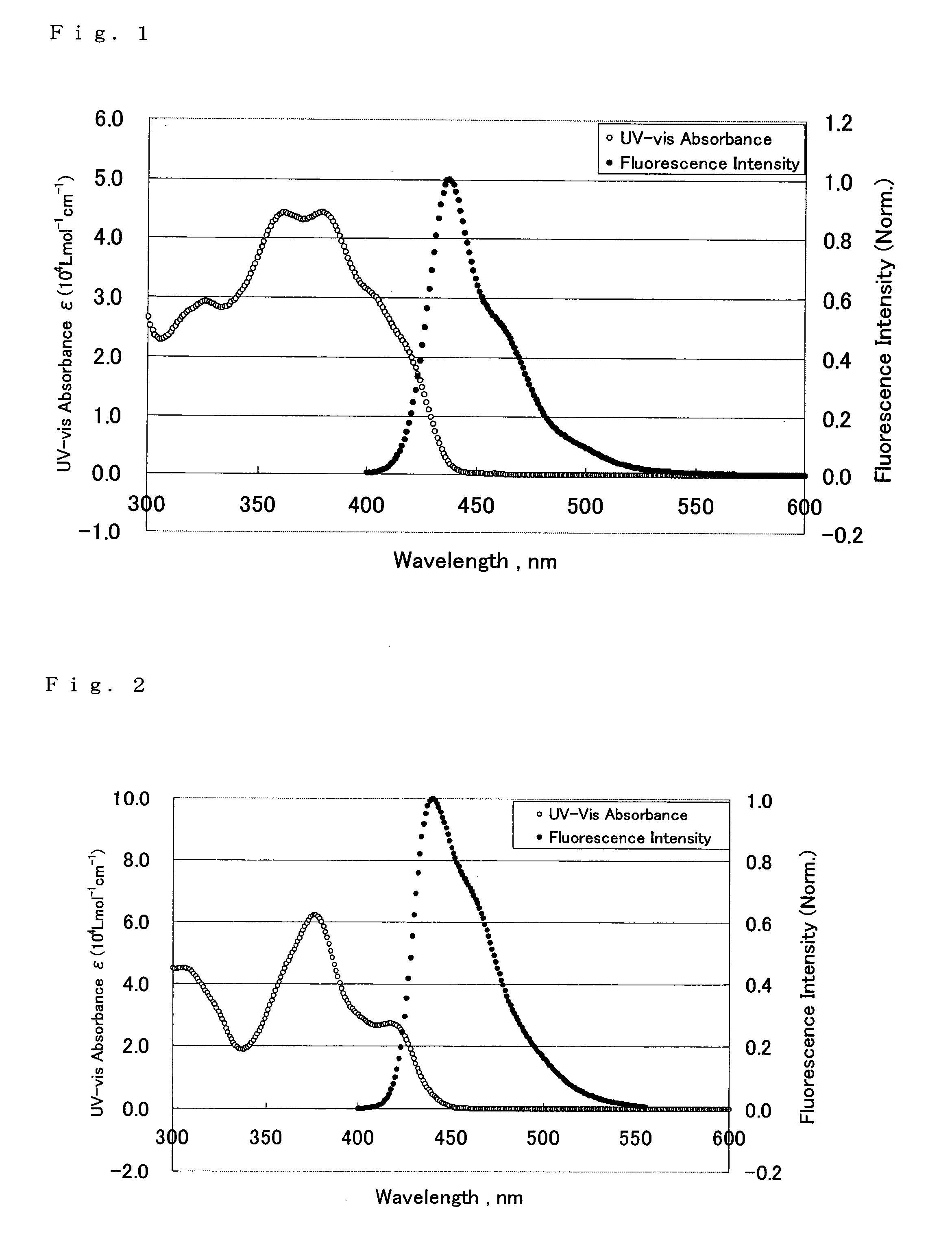
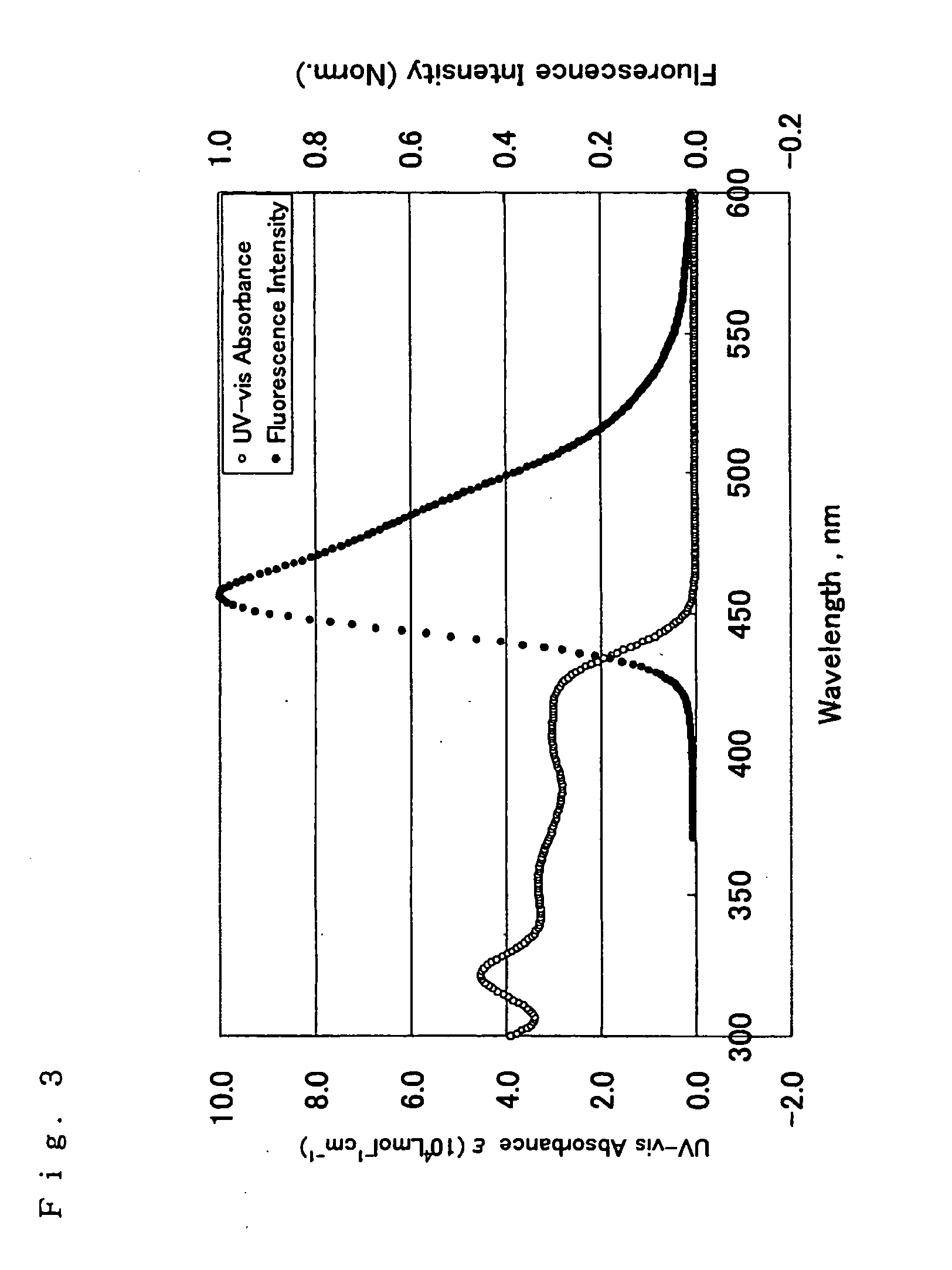
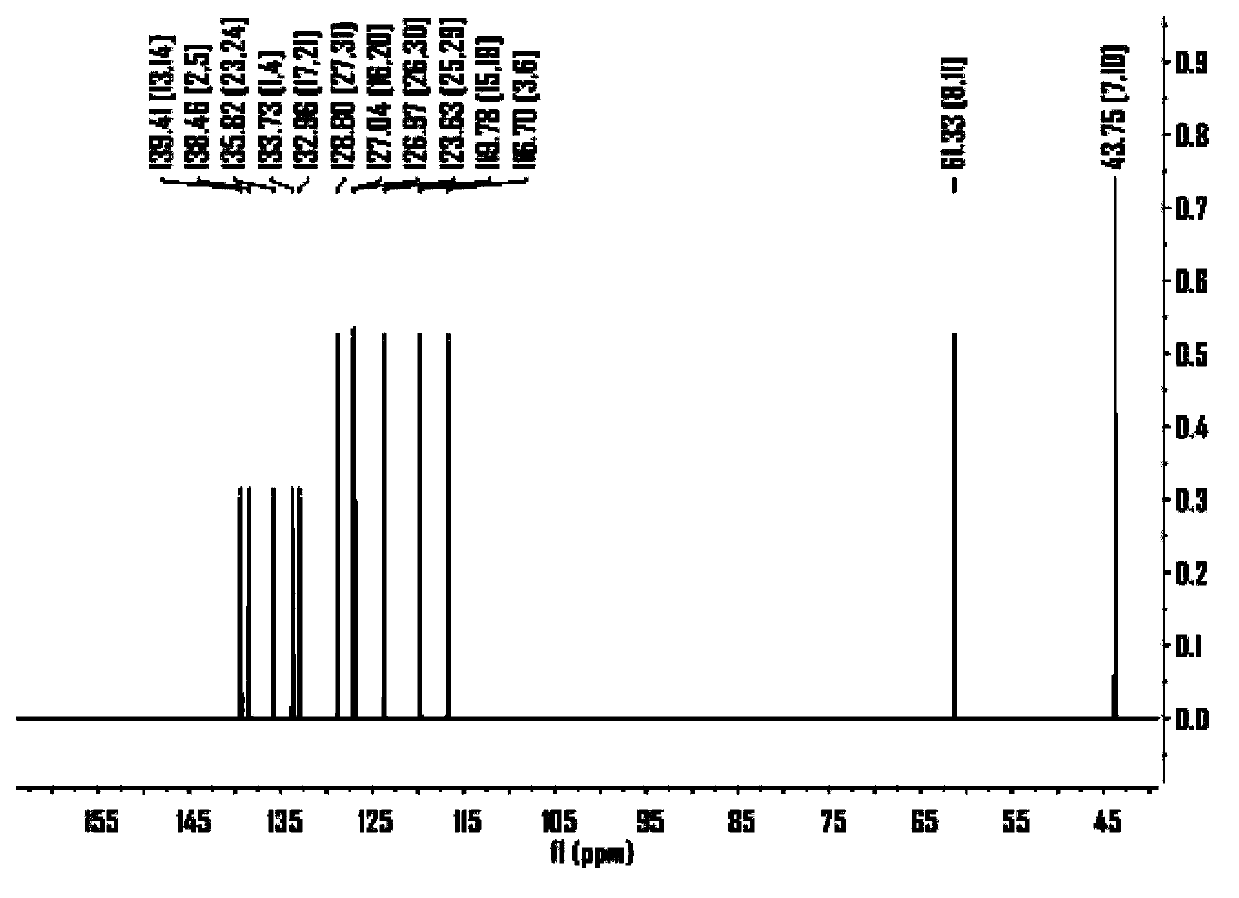
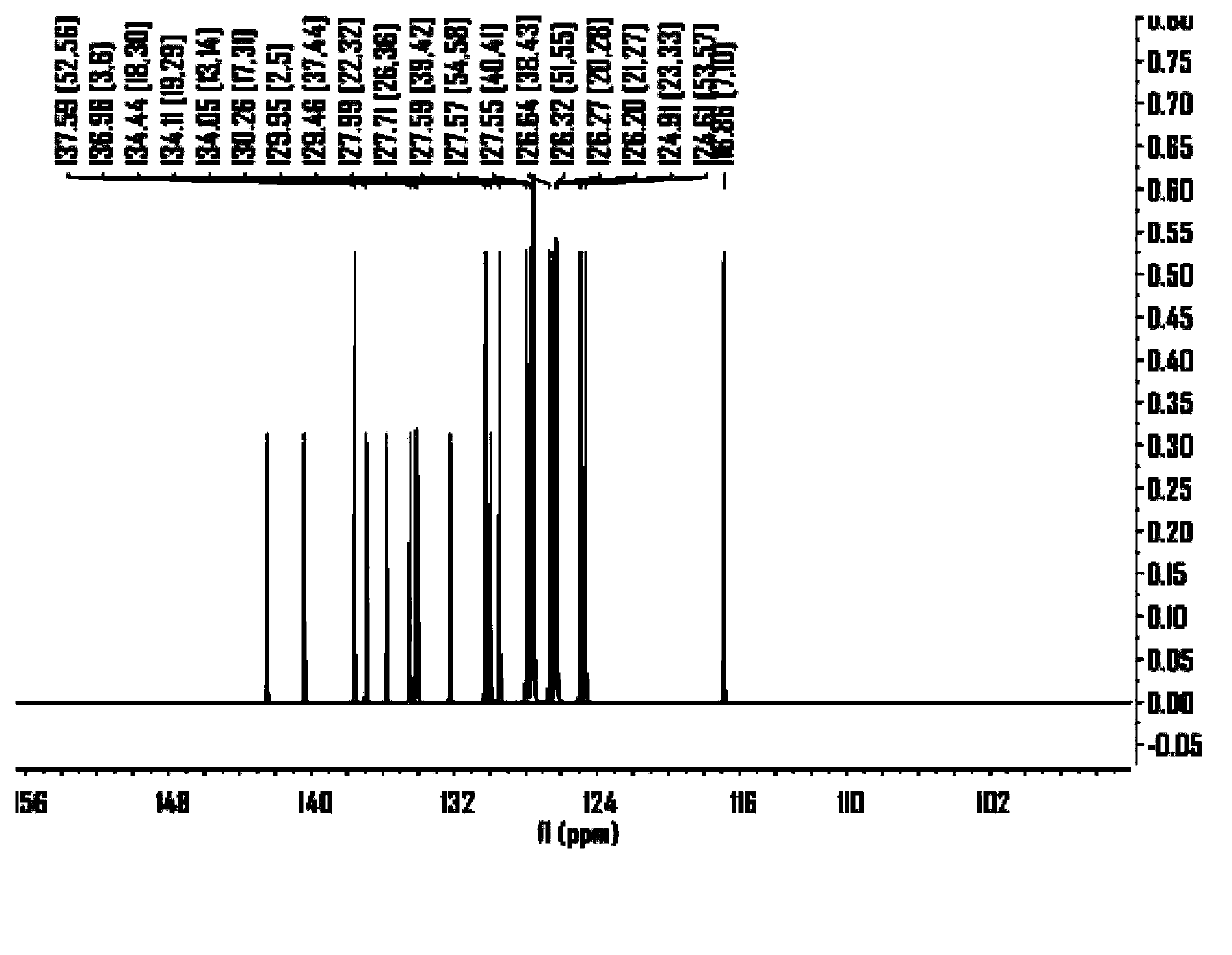
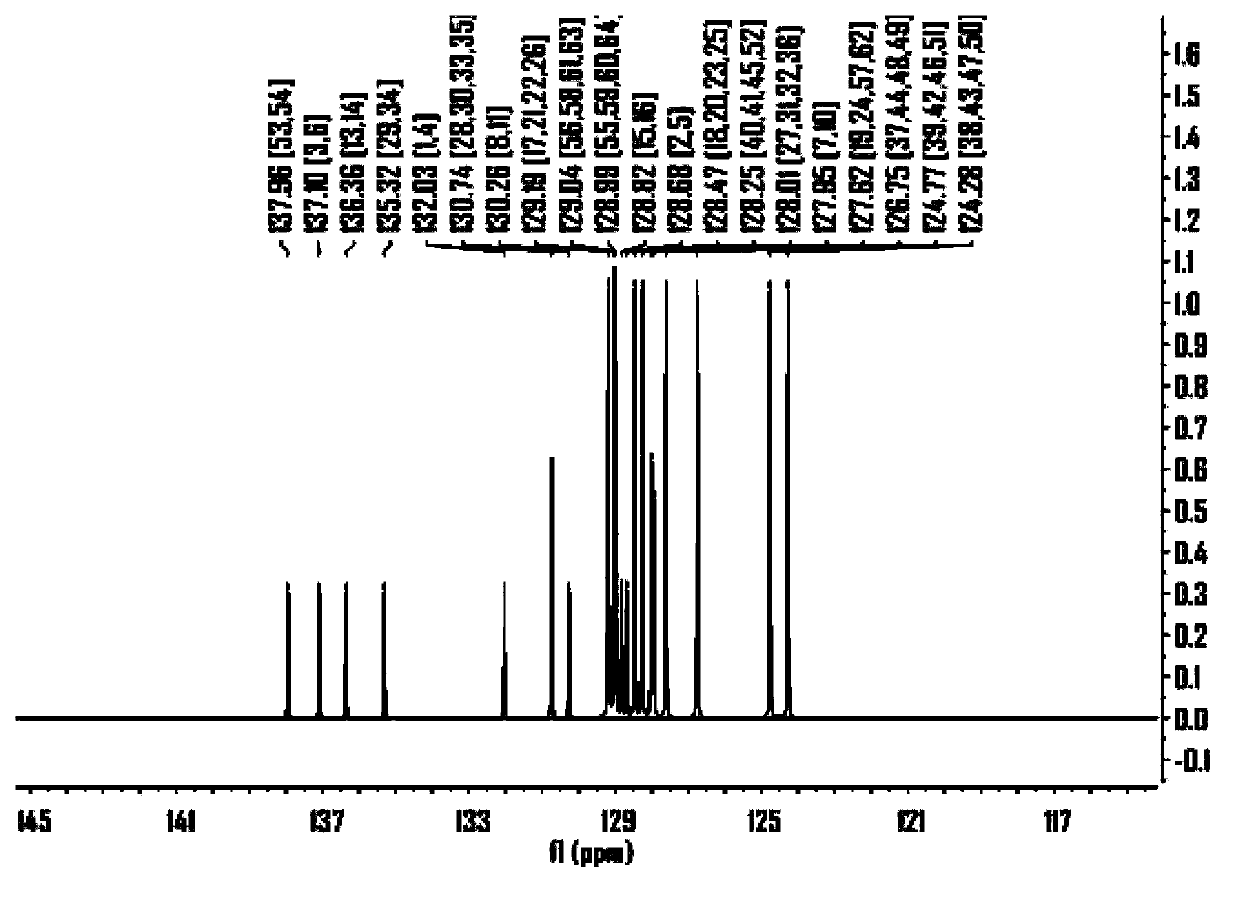
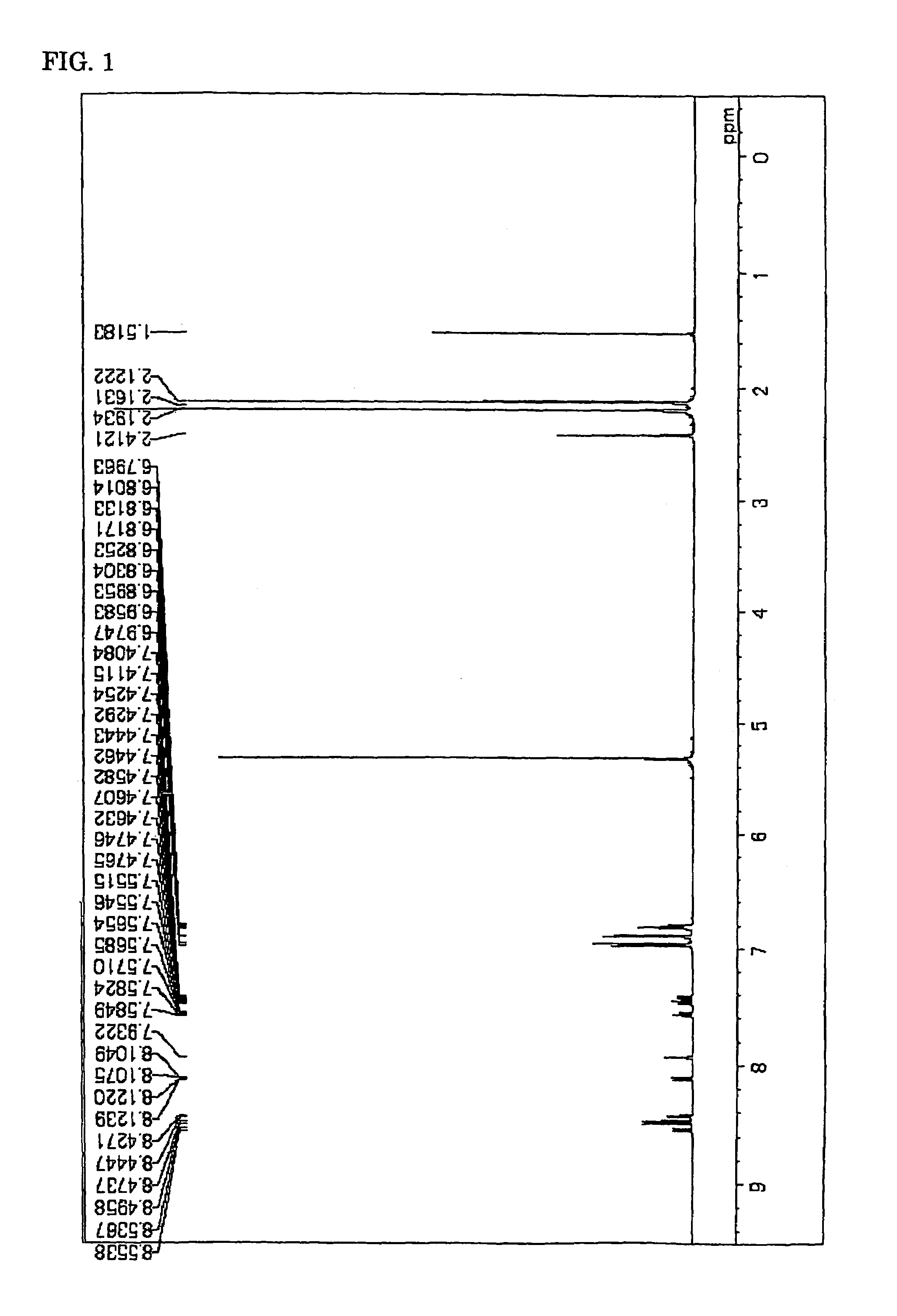
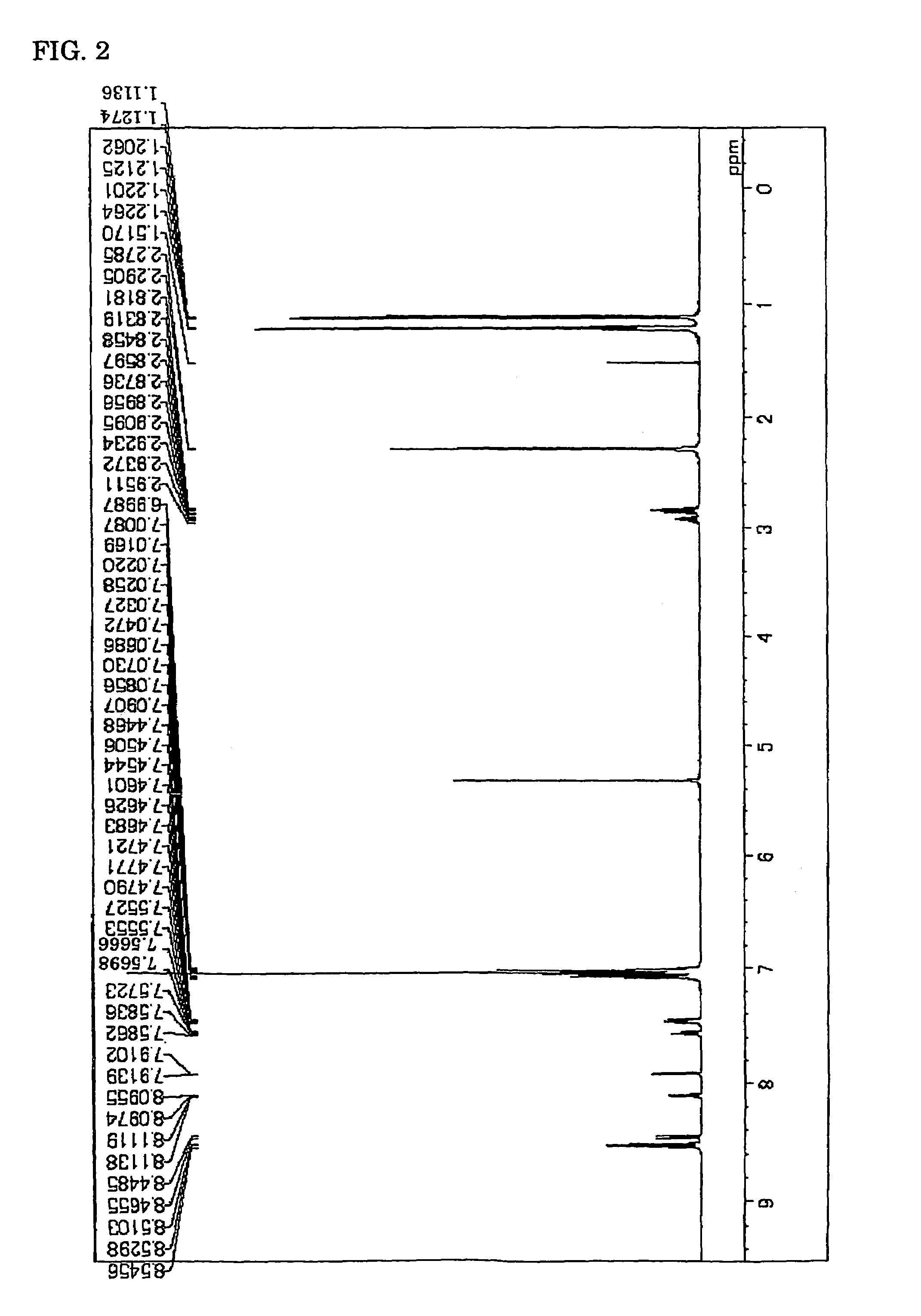
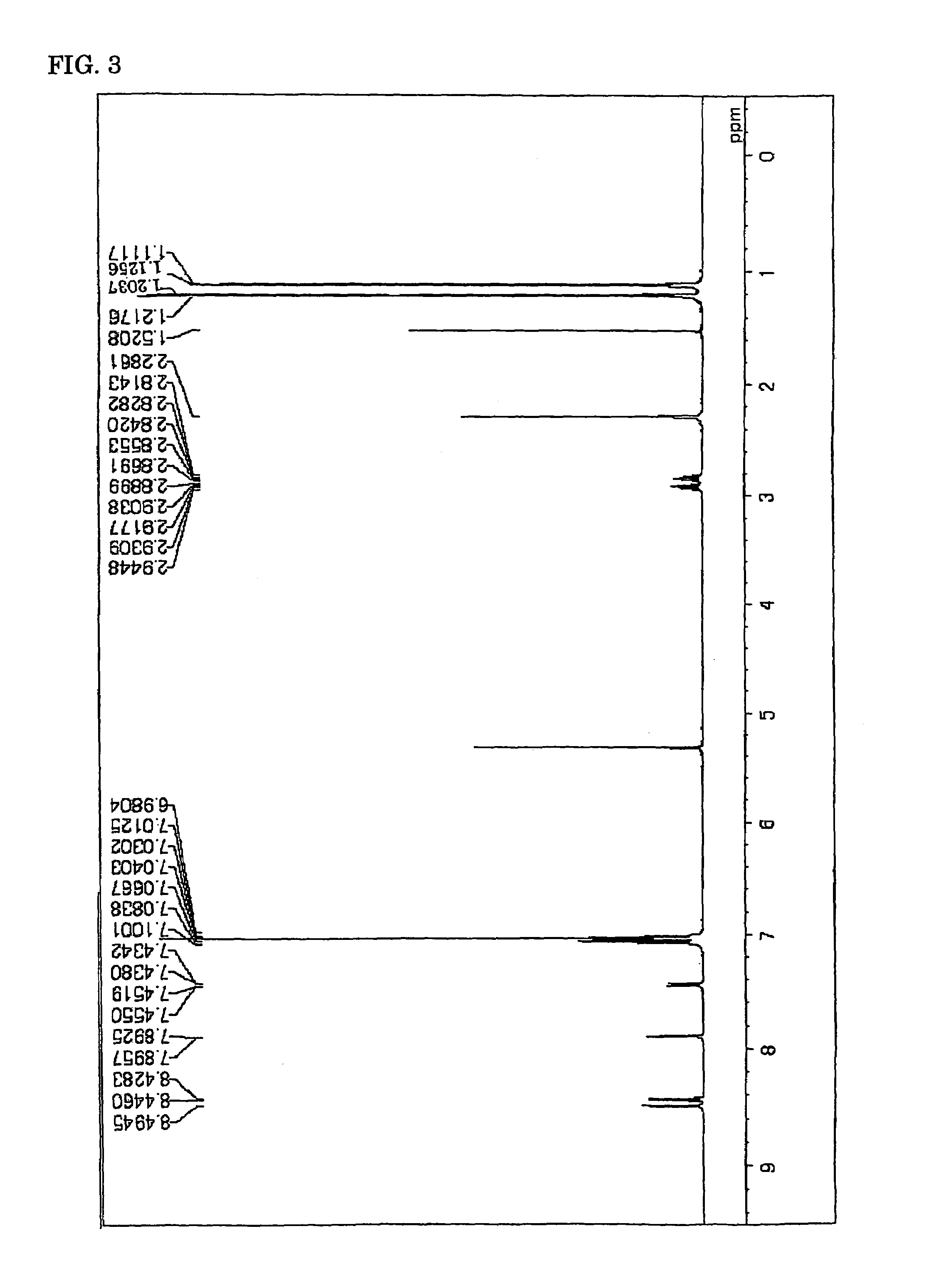
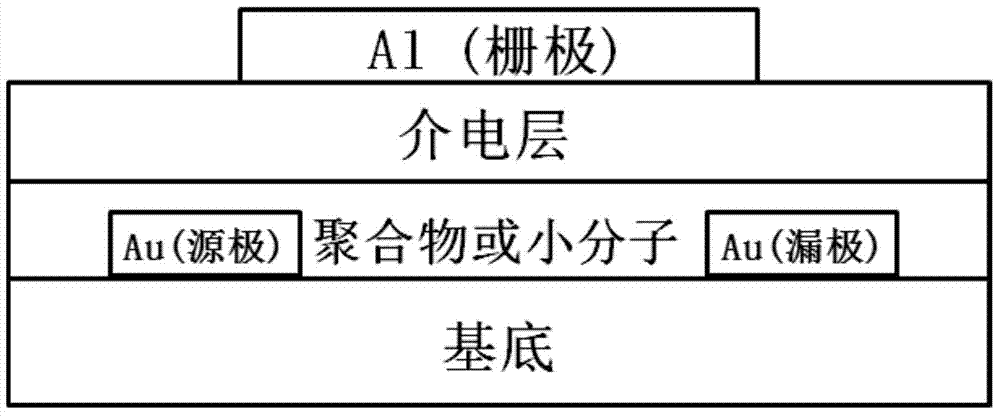
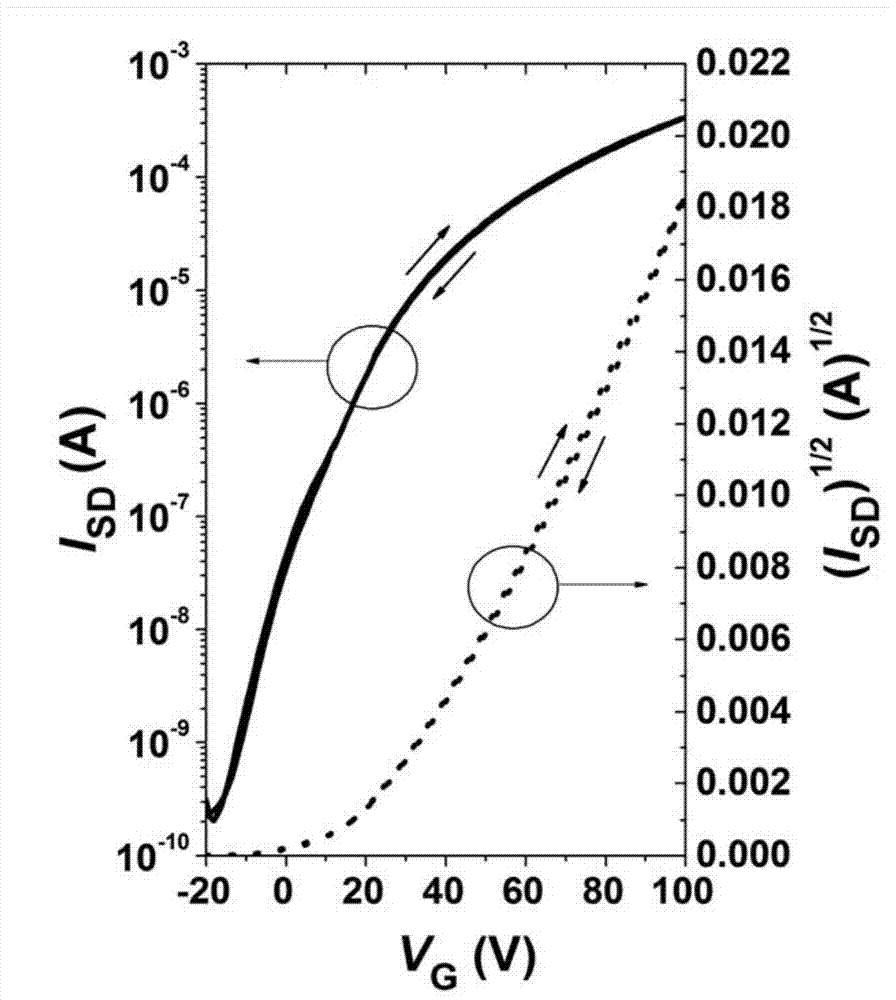
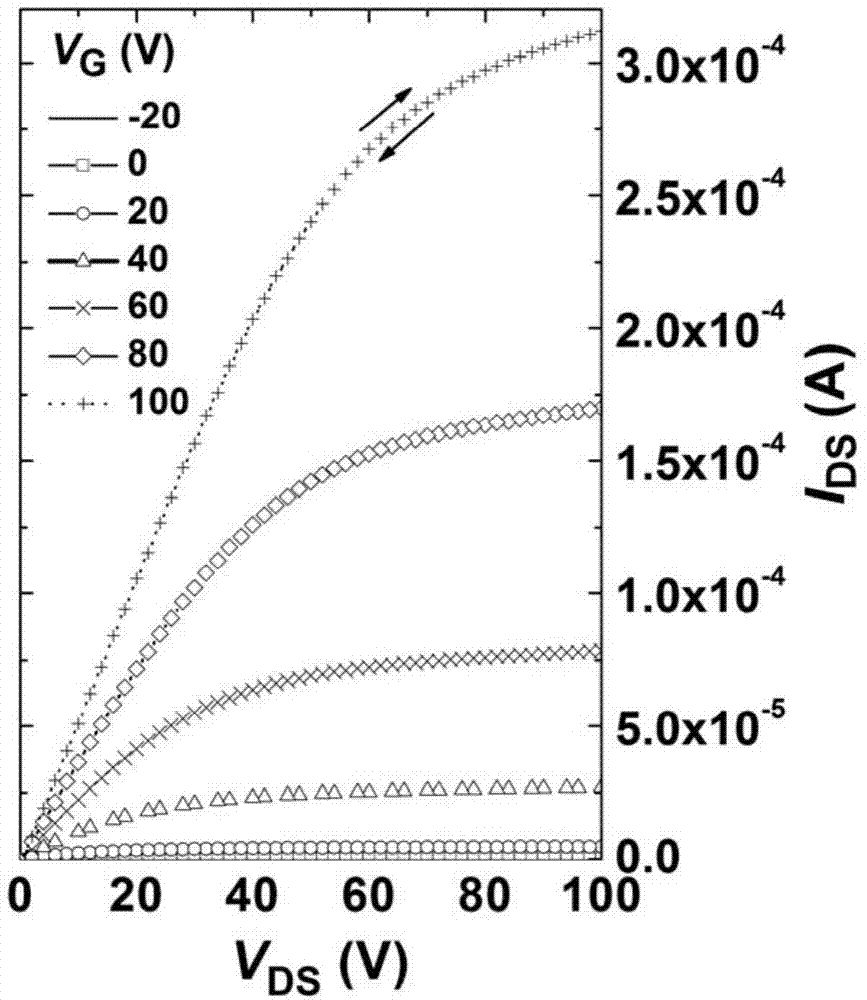
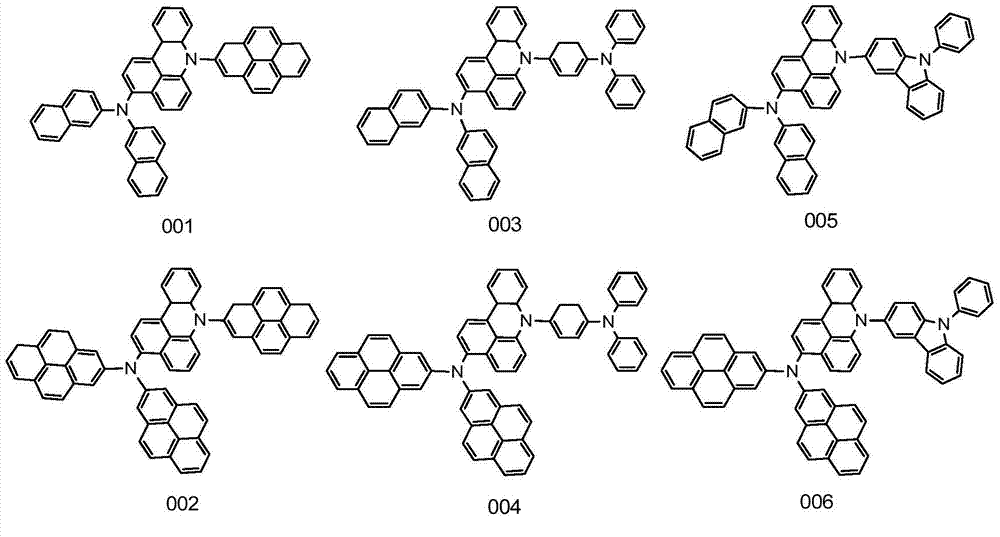
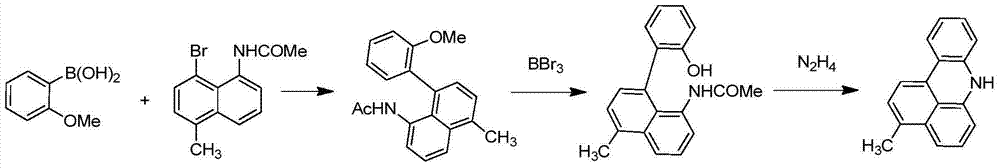
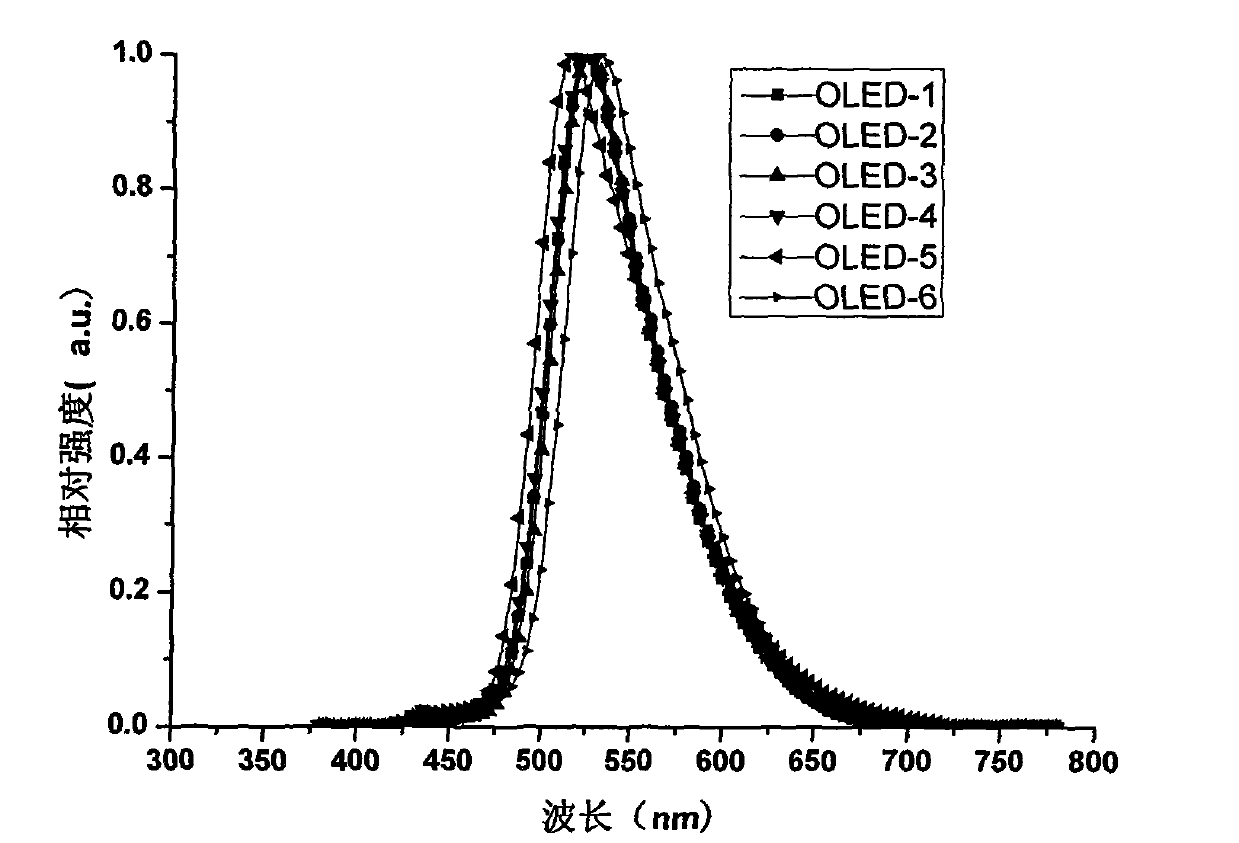
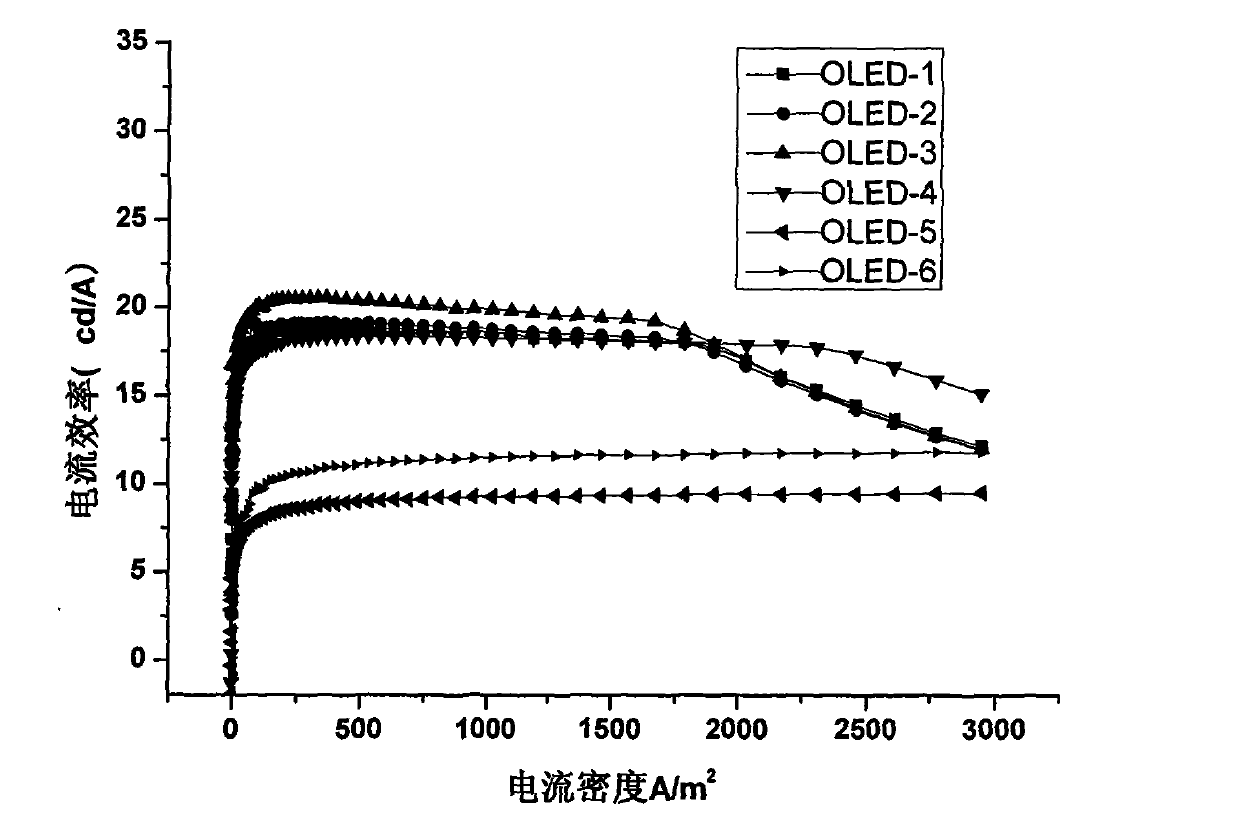
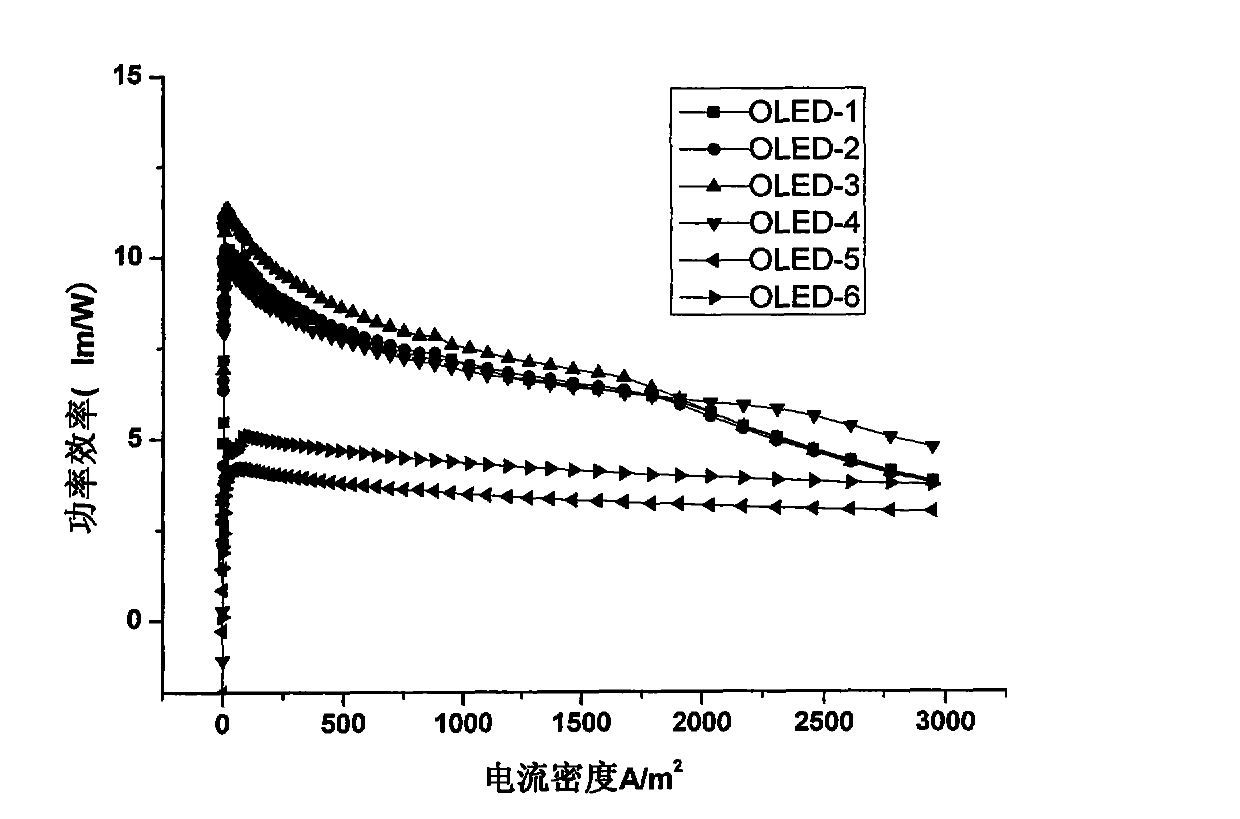
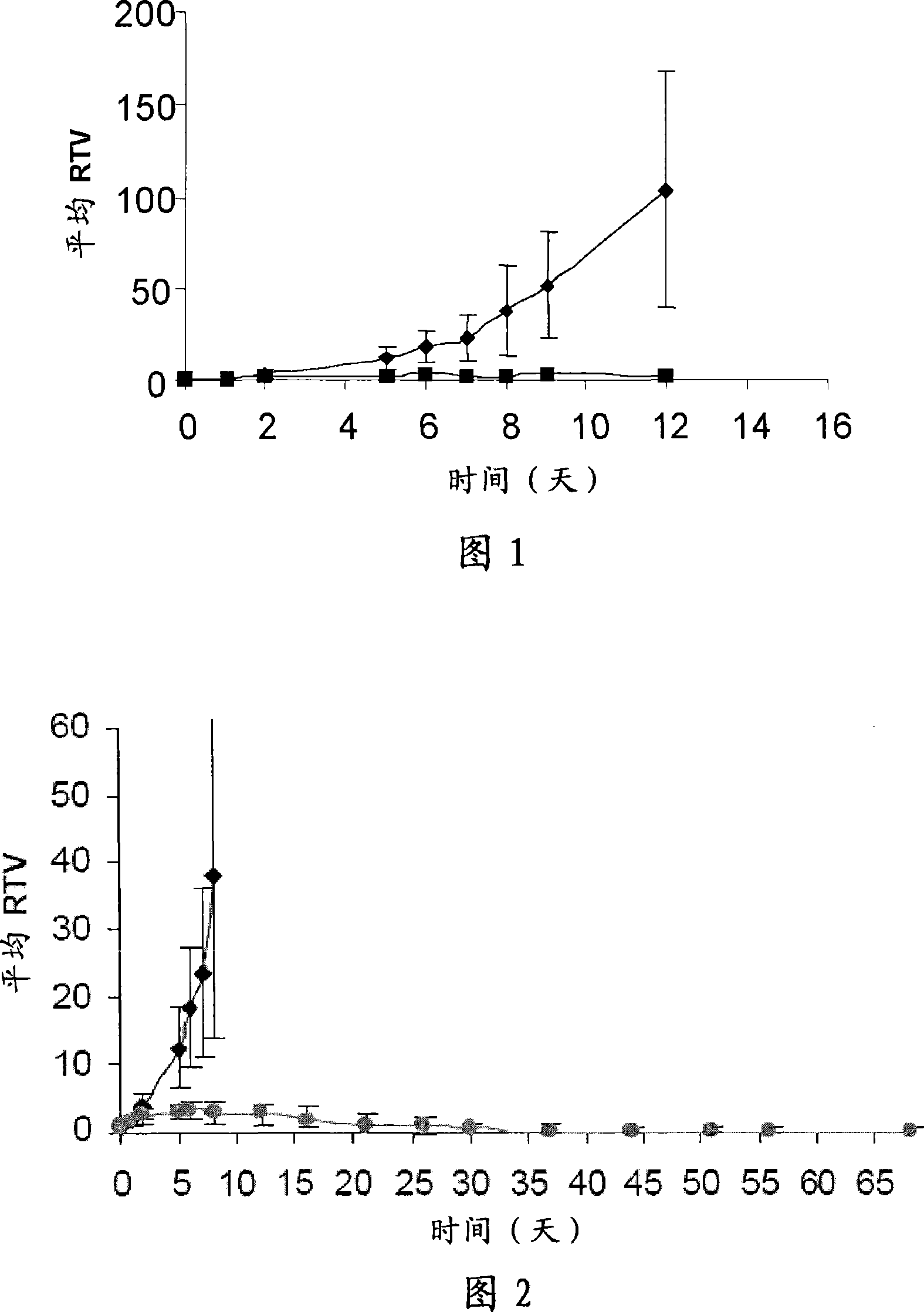
ActiveCN104649961AGood electron transport propertiesGood chemical and thermal stabilityOrganic chemistrySolid-state devicesCyclic compoundPhotochemistry
The invention relates to a kind of 2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds shown by formula (1) in the specification, wherein R1 and R2 are selected from C6-C50 aryl, substituted C6-C50 aryl or C1-C20 alkyl; or R1 and R2 are bonded through other groups to form a cyclic compound, Ar1 and Ar2 are separately selected from C5-C30 electron-deficient nitrogen-containing heterocyclic aromatic hydrocarbon, substituted nitrogen heterocyclic aromatic hydrocarbon or thick nitrogen-containing heterocyclic aromatic hydrocarbon or C6-C30 aromatic hydrocarbon or polycyclic aromatic hydrocarbon with the nitrogen-containing heterocyclic aromatic hydrocarbon substituent. The invention also provides an application of the compounds in an organic light emitting device (OLED) and particularly application of the compounds as an electron transport material and a fluorescent or red phosphorescence host material in the OLED device.
Owner:GUAN ETERNAL MATERIAL TECH
Removal of PCB and other halogenated organic contaminants found in ex situ structures
Emulsified systems of a surfactant-stabilized, biodegradable water-in-solvent emulsion with bimetallic particles contained with the emulsion droplets are useful at removing PCBs from ex situ structures. The hydrophobic emulsion system draws PCBs through the solvent / surfactant membrane. Once inside the membrane, the PCBs diffuse into the bimetallic particles and undergo degradation. The PCBs continue to enter, diffuse, degrade, and biphenyl will exit the particle maintaining a concentration gradient across the membrane and maintaining a driving force of the reaction.
Owner:NASA +1
Indene benzophenanthrene derivative and organic light-emitting device using same
ActiveCN103508835AShort half-lifeShort CIE Color PuritySolid-state devicesSemiconductor/solid-state device manufacturingCarbon atomAryl
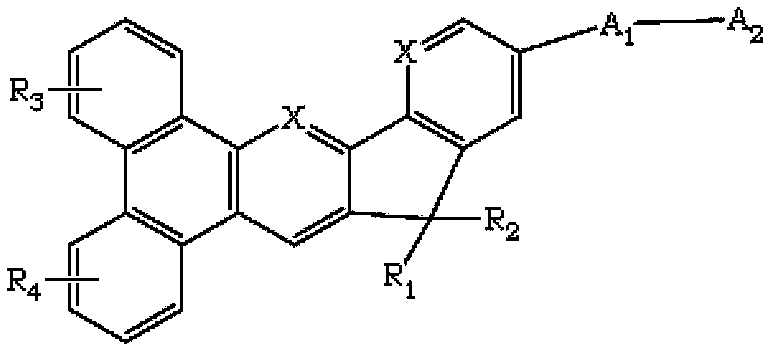
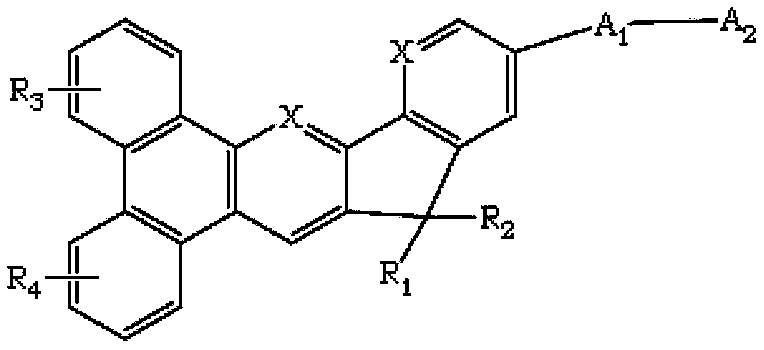
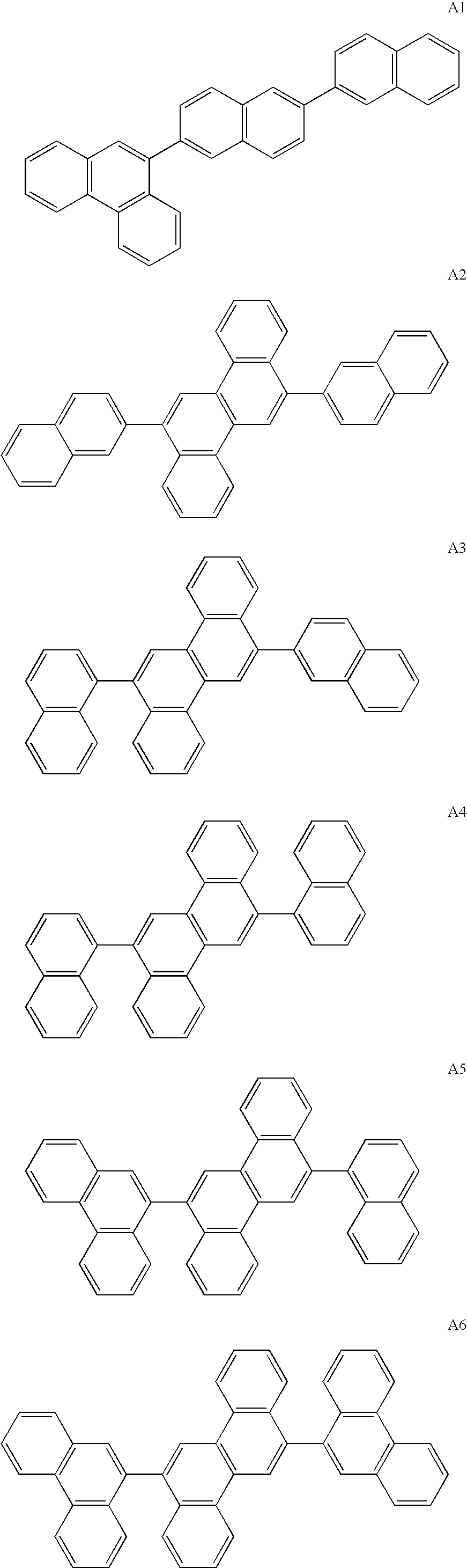
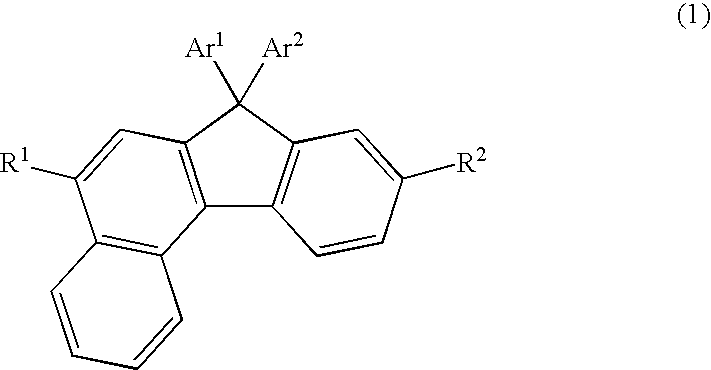
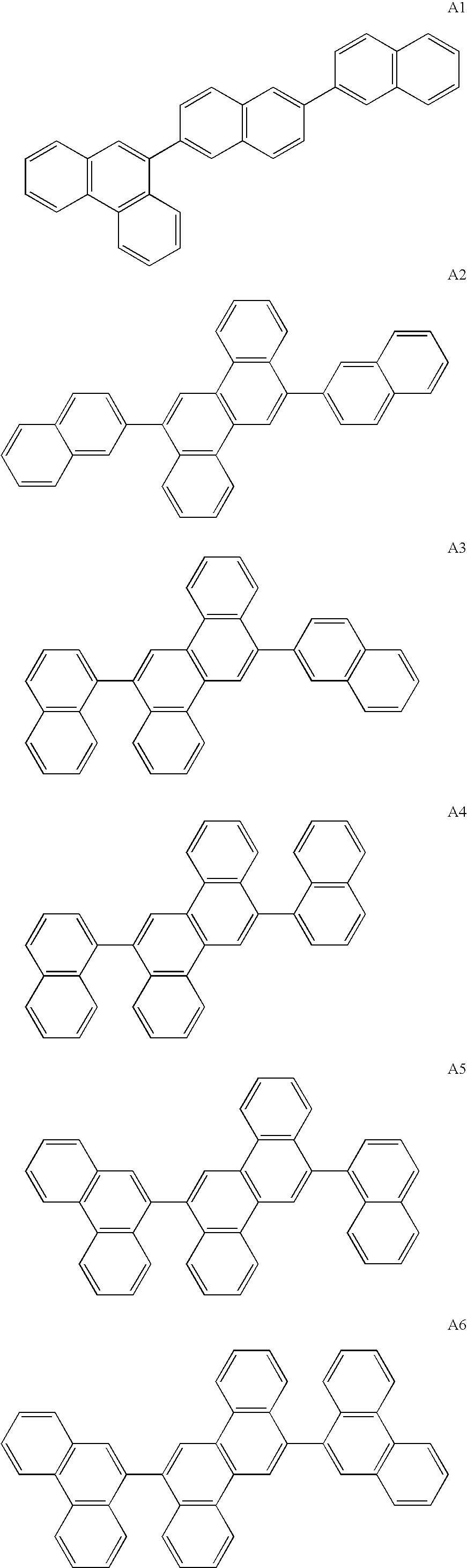
The invention discloses a novel indene benzophenanthrene derivative and an organic light-emitting device using same. The organic light-emitting device using the novel indene benzophenanthrene derivative as a host material can lower the driving voltage, prolong the half-life period, and improve the efficiency. The novel indene benzophenanthrene derivative is shown in the formula (A) in the specification: in formula (A), A1 and A2 are substituted or unsubstituted combined hydrocarbon ring units with 1-5 rings; preferably, A1 and A2 are phenyl, naphthyl, anthryl, pyrenyl, chrysene and perylene; R1-R4 are the same or different and are independently selected from hydrogen atom, halogen, alkyl group with 1-20 carbon atoms, substituted or unsubstituted aryl group with 6-30 carbon atoms, substituted or unsubstituted aralkyl with 6-30 carbon atoms, and substituted or unsubstituted ceteroary with 6-30 carbon atoms; and X can be carbon atom or nitrogen atom.
Owner:颜丰文
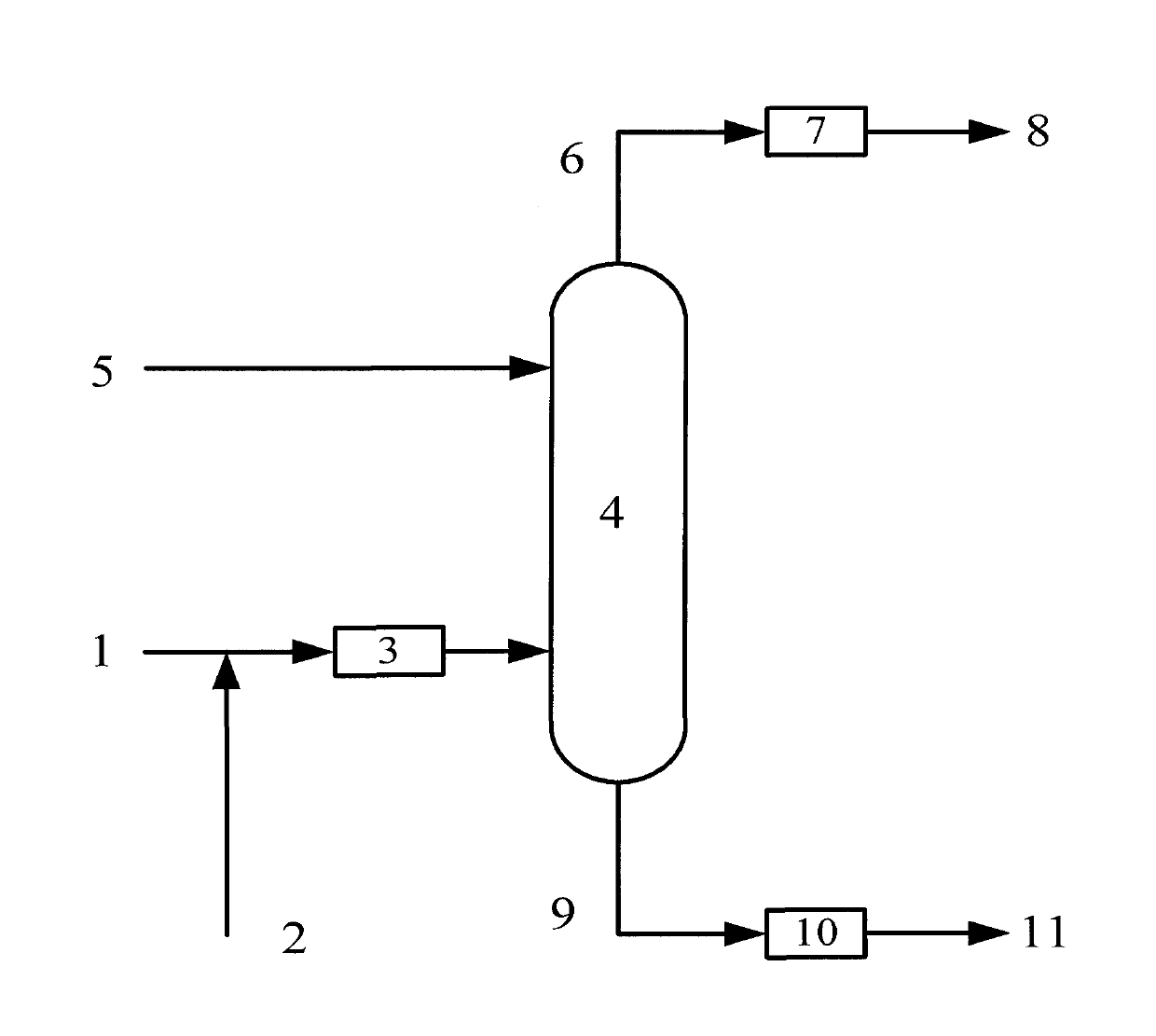
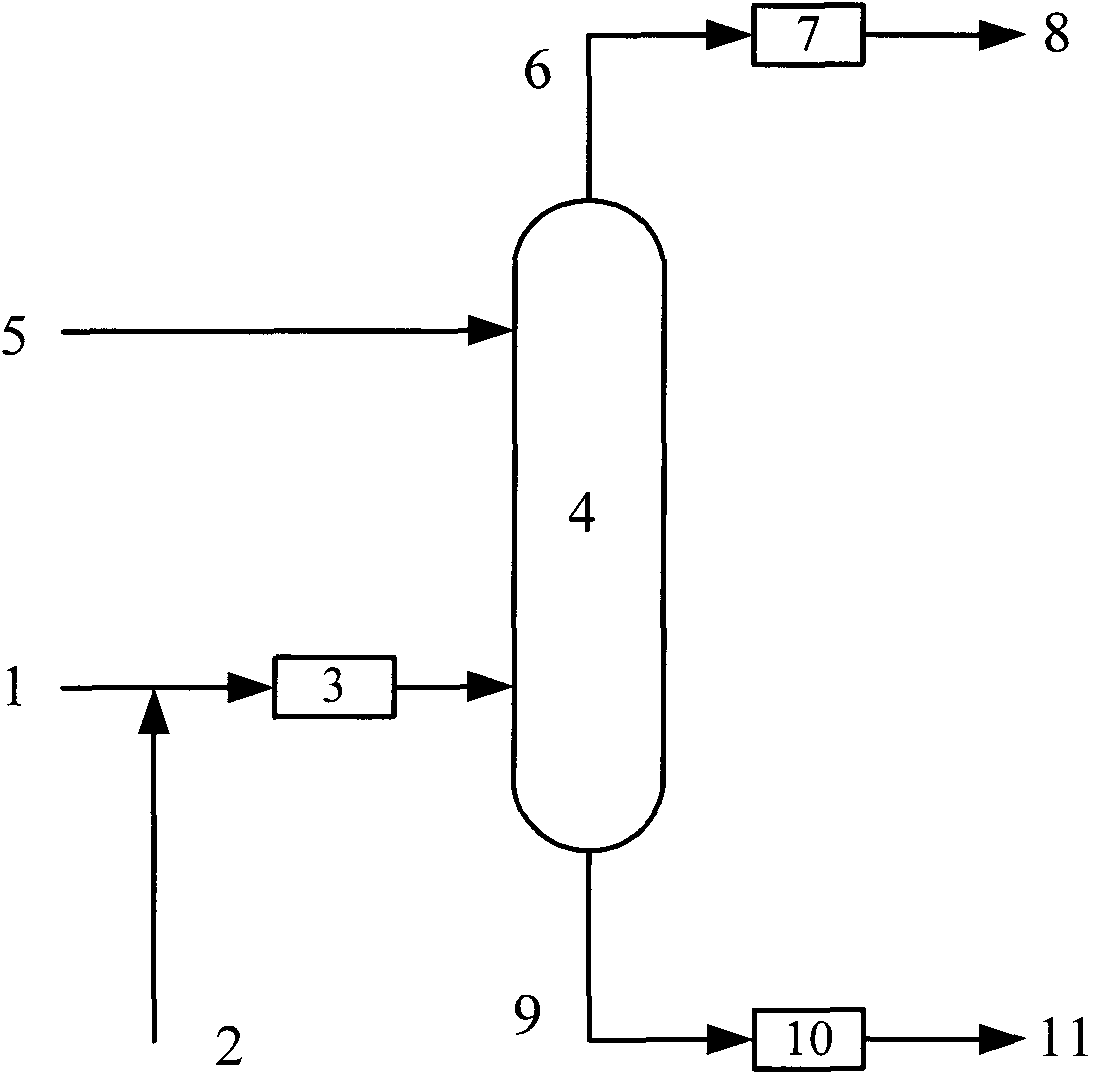
Preparation method for distillate aromatic extract (DAE)
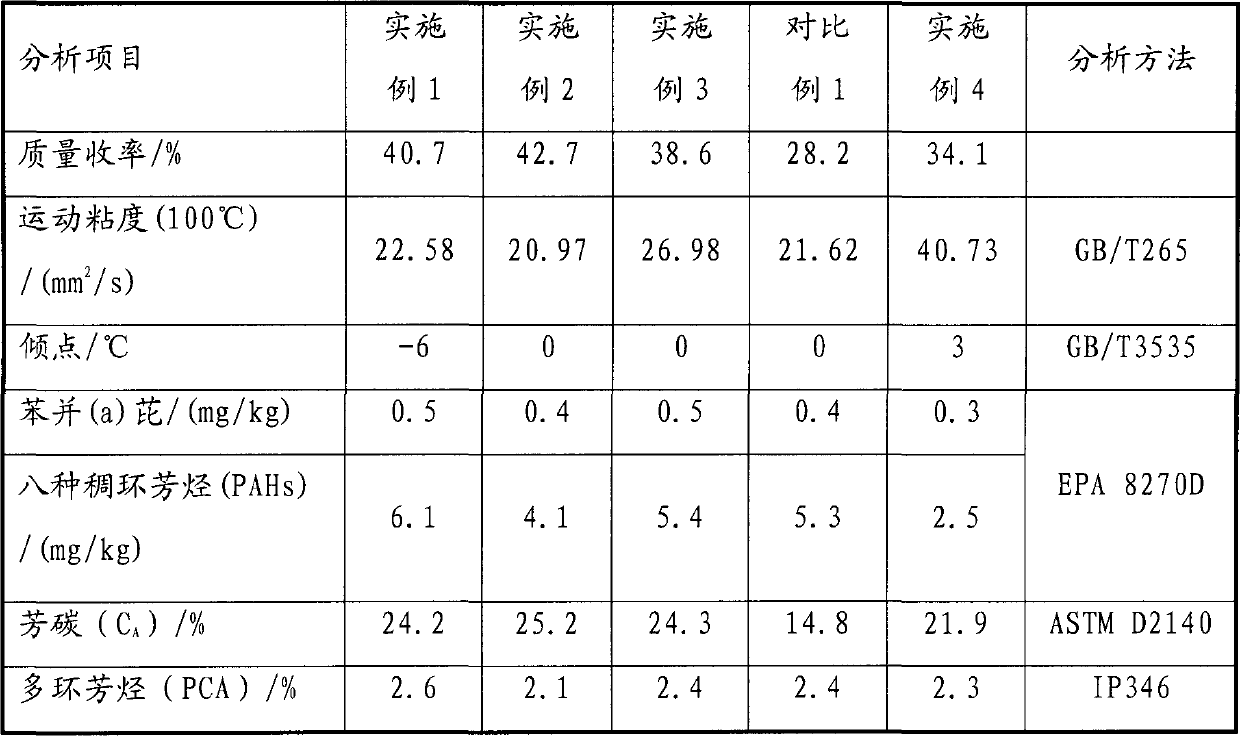
The invention relates to a preparation method for DAEs, comprising the following steps that: raw oil and a diluting solvent are mixed, the mixed solution enters into an extraction tower and contacts with an extraction solvent in the extraction tower, and raffinate oil is separated from raffinate, wherein the raw oil is extract oil obtained by solvent refining and / or de-waxing oil of the extract oil, the diluting solvent contains a main solvent, the extraction solvent contains the main solvent and an anti-solvent, and the main solvent has greater dissolvability of aromatic hydrocarbons than of alkane. According to the invention, the preparation method has high extraction efficiency; the extraction solvent used has good selectivity; DAE prepared by the method has high yield; the content of benzo(a)pyrene and the total content of 8 PAHs, namely, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(j)fluoranthene, benzo(a)pyrene, benzo(e)pyrene and dibenzo(a,h)anthracene, are low, which is in accordance with the European Union 2005 / 69 / EC instruction; the content of polycyclicaromatics is less than 3%.
Owner:CHINA PETROLEUM & CHEM CORP +1
Aminodibenzofluorene derivative and organic electroluminescence device using the same
ActiveUS20090267491A1Low working voltageLong life-timeGroup 4/14 element organic compoundsDischarge tube luminescnet screensOrganic electroluminescenceOrganic compound
A novel compound which is useful as a constitutional component for an organic EL device is provided by an aminodibenzofluorene derivative comprising (A) at least one dibenzofluorene structure and (B) at least one amino group in a molecule, a material for an organic electroluminescence (EL) device comprising the same, a light emitting material for an organic EL device, a light emitting organic solution, an organic EL device in which an organic compound layer comprising a single layer or plural layers including at least a light emitting layer is interposed between a pair of electrodes, wherein at leas one layer in the organic compound layer described above contains at least one kind of the aminodibenzofluorene derivative described above and an equipment comprising the same. A practical organic EL device which has a low operating voltage, a long lifetime and a high current efficiency and which provides blue light emission having an excellent color purity is materialized by using the above compound.
Owner:IDEMITSU KOSAN CO LTD
Benzodithiophene derivative organic electroluminescent material and application thereof
InactiveCN103664994AHigh hole mobilityOrganic chemistrySolid-state devicesFluorescenceOrganic electroluminescence
The invention provides novel compounds, of which the structures are represented as Formula (I), Formula (II), Formula (III) and Formula (IV), wherein Ar1-Ar6 are selected from C1-C20 aliphatic alkyl groups, C4-C30 aromatic rings, C4-C30 aromatic heterocyclic rings, C4-C30 condensed heterocyclic ring aromatics, C4-C30 arylamino or triarylamino groups or C4-C30 aryloxy groups. The compounds are used as a hole injection material, hole transmission material or fluorescence body material in organic electroluminescent devices.
Owner:KUNSHAN VISIONOX DISPLAY TECH +2
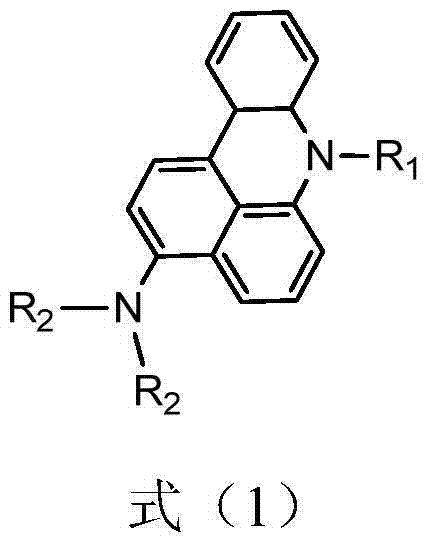
Aromatic amine derivative and organic electroluminescence device employing the same
ActiveUS7425653B2Long lastingImprove efficiencyOrganic chemistryElectroluminescent light sourcesOrganic electroluminescenceLight emission
A specified aromatic amine derivative having a chrysene structure. An organic electroluminescence device which comprises at least one organic thin film layer comprising a light emitting layer sandwiched between a pair of electrode consisting of an anode and a cathode, wherein at least one of the organic thin film layer comprises the aromatic amine derivative singly or as its mixture component. Organic electroluminescence devices having a long lifetime and a high efficiency of light emission, and aromatic amine derivatives capable of realizing such organic electroluminescence devices are provided.
Owner:IDEMITSU KOSAN CO LTD
Photo acid generator compounds, photo resists, and method for improving bias
InactiveUS6074800AEasy to controlImprove thermal stabilityOrganic chemistryOrganic compound preparationResistSulfonate
Several mid UV photo acid generators (PAGs), a chemically amplified photo resist (CAMP), and method for improving nested to isolated line bias are provided. Similarly, photo speed may also be improved. Unlike conventional mid UV PAGs, the present invention's PAG compounds, resist composition, and method do not require a mid UV sensitizer. Specifically, PAGs are provided that bear a chromophore capable of receiving mid UV radiation, particularly I-line, and that are suitable for use in a chemically amplified photo resist having a photo speed of 500 mJ / cm2 or less, but preferrably 200 mJ / cm2 or less. For example, the PAGs can be a sulfonium or iodonium salt, such as anthryl, butyl, methyl sulfonium triflate and bis(4-t-butylphenyl)iodonium 9,10-dimethoxyanthracene sulfonate. The chromophore forming a part of the PAGs can be selected from polyaromatic hydrocarbons, for example, chrysenes, pyrenes, fluoranthenes, anthrones, benzophenones, thioxanthones, anthracenes, and phenanthrenes, but preferably anthracenes.
Owner:GLOBALFOUNDRIES INC
Electron withdrawing group-containing phenylene vinylene compound, and preparation method and application thereof
InactiveCN104761563AEasy to convertHigh hole mobilityOrganic chemistrySolid-state devicesOrganic solar cellSemiconductor materials
The invention discloses an electron withdrawing group-containing phenylene vinylene compound, and a preparation method and an application thereof. The electron withdrawing group-containing phenylene vinylene compound monomer or polymers obtained by polymerizing the monomer and other aromatic compound monomers greatly improve the hole and electron mobility in a material as an organic semiconductor material due to the containing of a electron withdrawing substituent group, so the monomer or the polymers can be used in organic field effect transistors, organic solar batteries, organic light-emitting diodes and other photoelectric devices as an n-type material (for transferring electrons) or a bipolar transfer material (for transferring holes and electrons).
Owner:PEKING UNIV
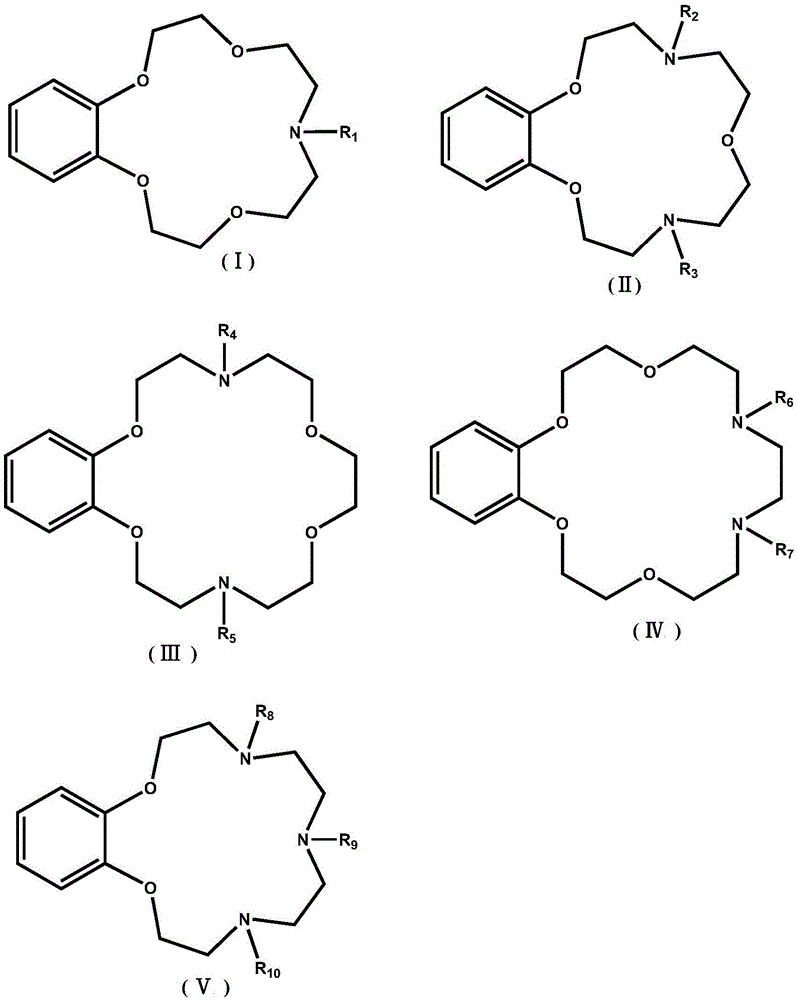
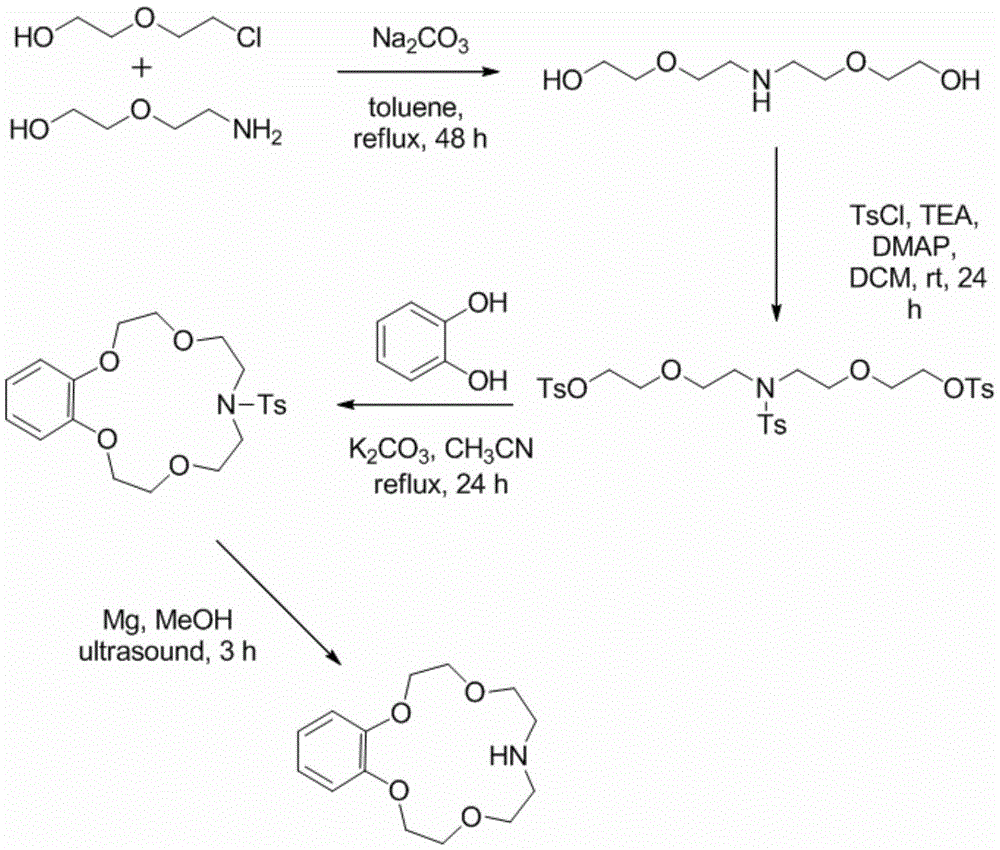
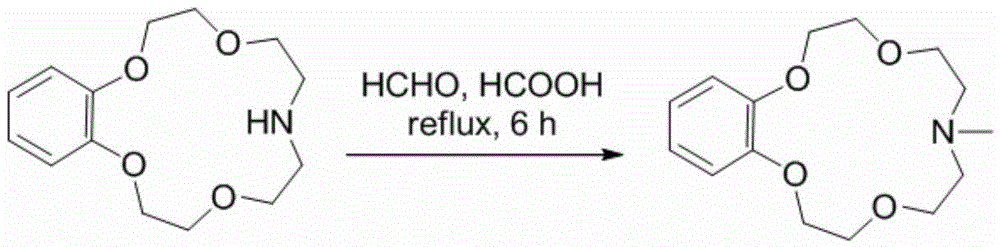
Application of benzo-azacrown ether compounds to separation of lithium isotopes
ActiveCN105561790AEfficient separationFast exchange rateOrganic chemistryIsotope separationIsotope18-Crown-6
The invention discloses application of benzo-azacrown ether compounds to separation of lithium isotopes. The benzo-azacrown ether compounds are selected from monoaza15-crown-5, bisaza15-crown-5, triaza15-crown-5 and bisaza18-crown-6. The benzo-azacrown ether compounds serving as extracting agents are dissolved in organic solvents to prepare organic phases, a lithium trifluoroacetate aqueous solution serves as an aqueous phase, and the lithium isotopes are separated at the room temperature by liquid-liquid extraction. The benzo-azacrown ether compounds are easy to dissolve in the organic solvents, efficient separation of the lithium isotopes can be realized by means of liquid-liquid extraction, and considerable separation factors, simplicity and convenience in operation, quickness in isotope exchange and technical simplicity are realized.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Green organic electroluminescent material and preparation method thereof
ActiveCN103589420AStrong fluorescence emission functionRegulatory transitionOrganic chemistryLuminescent compositionsBromineMethyl group
The invention relates to a green organic electroluminescent material and a preparation method thereof, and solves the technical problem that the luminous efficiency of an existing luminous material still cannot meet the requirements of OLEDs (organic light-emitting diodes). According to the green organic electroluminescent material, 3-methyl-7H-benzacridine and R1 bromide are taken as raw materials and react to obtain a benzacridine compound containing an R1 substituent group, the benzacridine compound is subjected to a reducing process by palladium carbon to obtain a benzacridine amine compound containing the R1 substituent group, and the benzacridine amine compound continues to react with R2 bromide to obtain a benzacridine amine compound containing R1 and R2 substituent groups. Due to introduction of different substituent groups, electron transition can be adjusted to adjust luminous peak so as to obtain the required green organic electroluminescent material. According to the preparation method provided by the invention, synthesis and purification processes are simpler, the cost is low, and the industrialization development requirements can be met.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
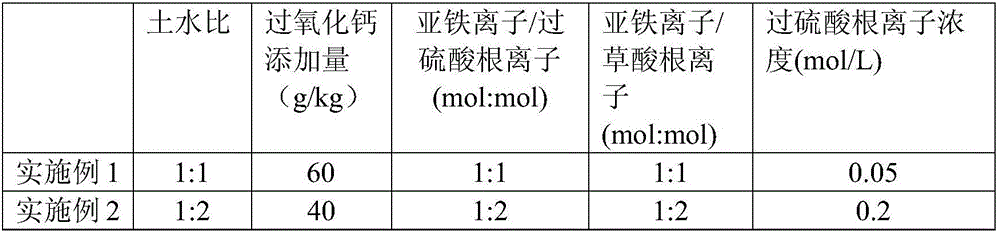
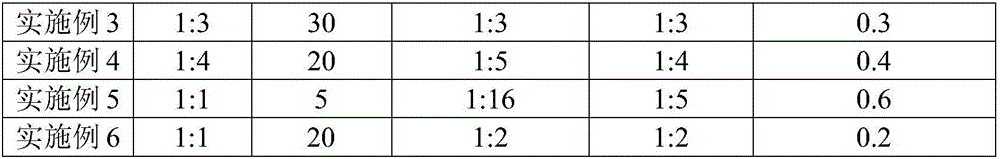
Method for removing polycyclic aromatic hydrocarbons in soil by using persulfate-calcium peroxide through compound oxidation
ActiveCN106345800AMaintain a neutral pHHigh removal rateContaminated soil reclamationAcenaphthylenePeroxydisulfate
The invention discloses a method for removing polycyclic aromatic hydrocarbons in soil by using persulfate-calcium peroxide through compound oxidation, and belongs to the field of soil repairing. The method comprises the following steps: after sieving to-be-measured air-dried soil, mixing the soil with calcium peroxide evenly; then adding distilled water to obtain slurry; successively adding oxalate ions, ferrous ions and peroxydisulfate ions, and stirring uniformly to obtain slurry reaction liquid; then leaving the slurry reaction liquid standstill in a dark place so that the polycyclic aromatic hydrocarbons in the soil can be removed after reaction is finished. By the method, naphthalene, acenaphthylene, acenaphthene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, a component as shown in the specification, benzo[b] fluoranthene, benzo[a]pyrene, dibenzo[a,b]anthracene, benzo[ghi] perylene and indeno[1,2,3-cd] pyrene in a complicated environment of the soil can be removed effectively, the removal rate of the polycyclic aromatic hydrocarbons is high, neutral pH of the soil can be maintained, acidized soil is improved, and a catalyst and a chelating agent are adopted, so that the removal effect and the removal efficiency are enhanced.
Owner:NANJING AGRICULTURAL UNIVERSITY
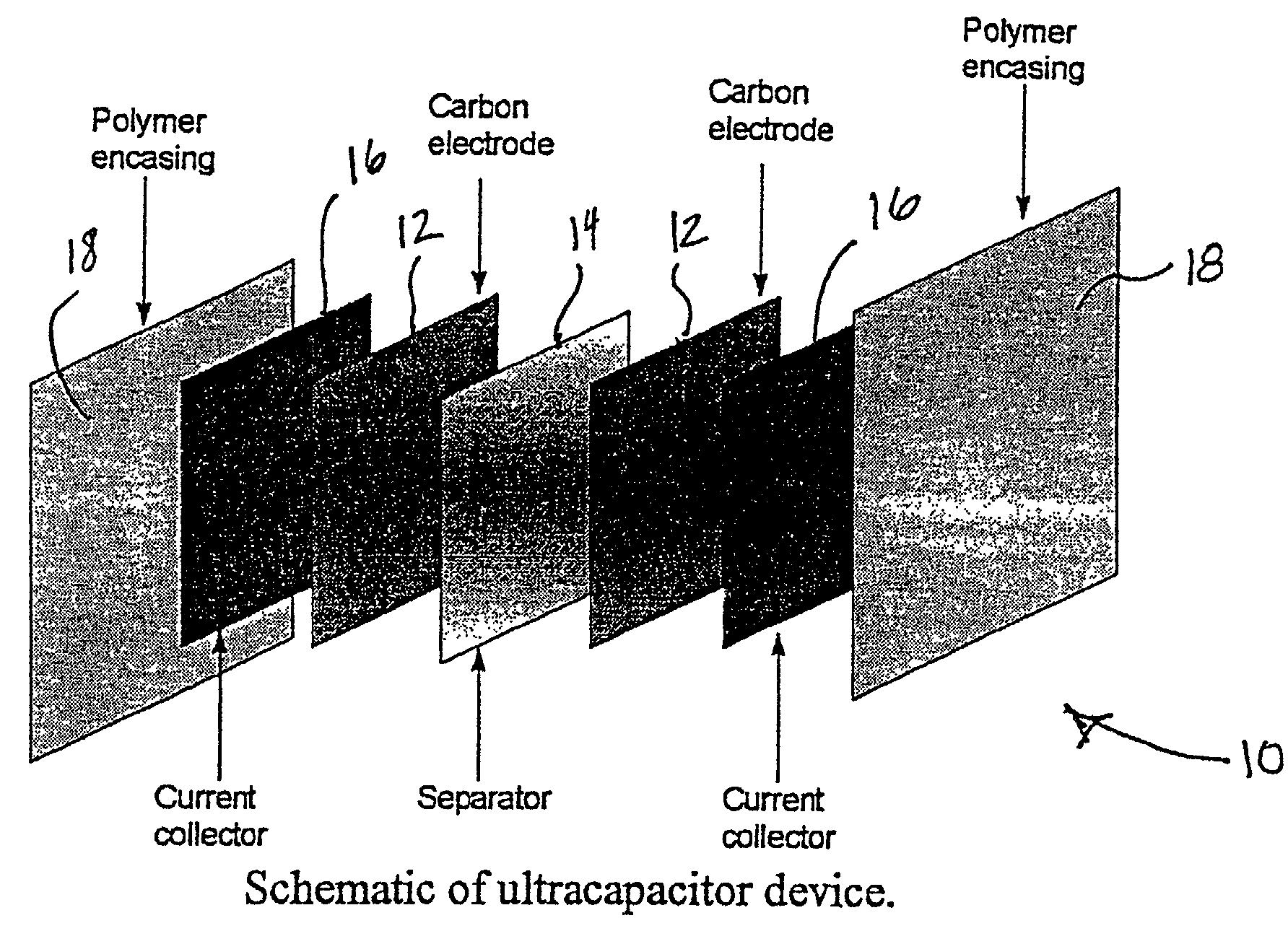
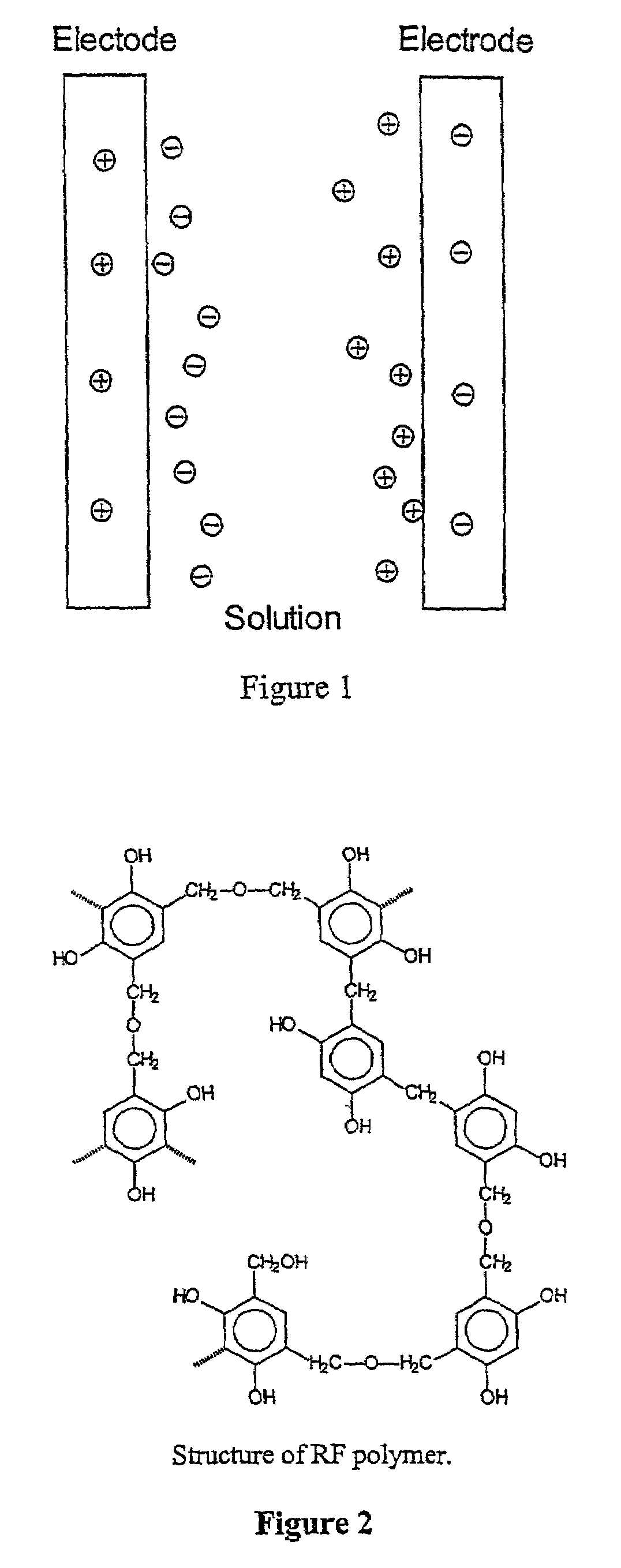
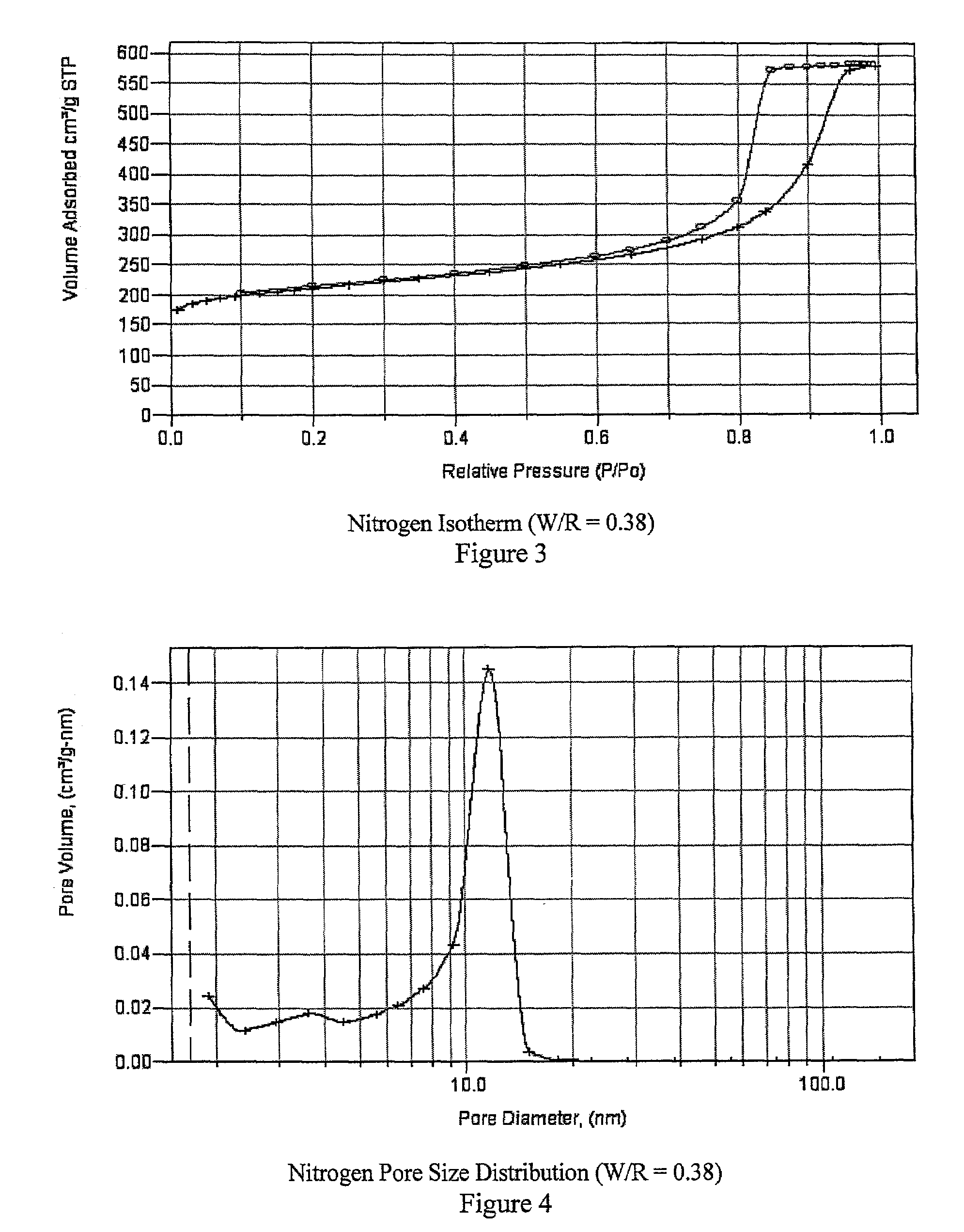
Mesoporous carbons and polymers from hydroxylated benzenes
InactiveUS7167354B2High mesoporositySimple and inexpensive to manufactureHybrid capacitor electrolytesPhysical/chemical process catalystsPolymer scienceSorbent
A mesoporous polymer and method of preparing a mesoporous polymer whose polymerization kinetics are dependent upon pH and whose pore size is controlled by pH and solvent concentration are disclosed. The polymer is optionally pyrolyzed to form a primarily carbonaceous solid. The material has an average pore size in the mesopore range and is suitable for use in liquid-phase surface limited applications including chromatographic, sorbent, catalytic, and electrical applications.
Owner:TDA RES
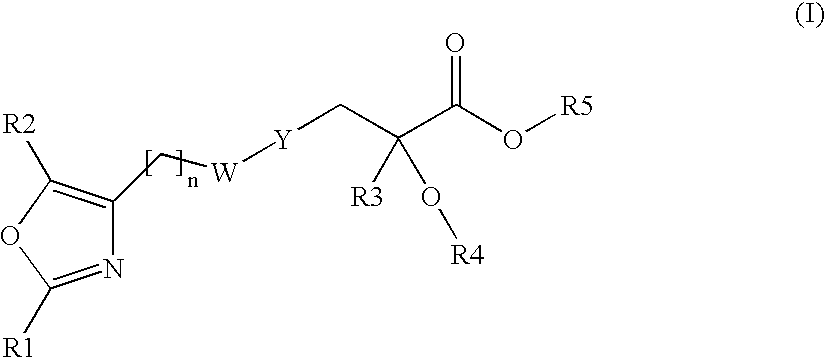
Oxazolyl-arylproplonic acid derivatives and their use as ppar agonists
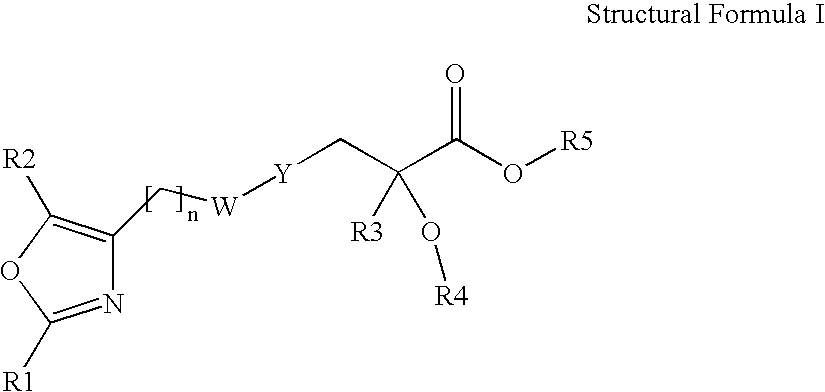
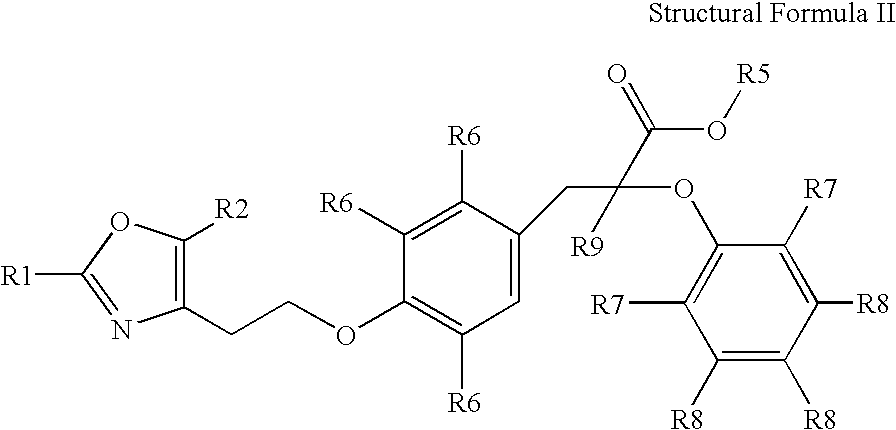
Compounds represented by the following structural formula (I), and pharmaceutically acceptable salts, solvates and hydrates thereof, wherein: n is 2, 3, or 4 and W is CH2, CH(OH), C(O) or O; R1 is an unsubstituted or substituted aryl, heteroaryl, cycloalkyl, heterocycloalkyl, aryl-alkyl, heteroaryl-alkyl, cycloalkyl-alkyl, or t-butyl; R2 is H, alkyl, haloalkyl or phenyl; Y is an unsubstituted or substituted thiophen-2,5-diyl or phenylene; R3 is alkyl or haloalkyl; R4 is a substituted or unsubstituted phenyl, naphthyl, 1,2,3,4-tetrahydronaphthyl, quinolyl, pyridyl or benzo[1,3]dioxol-5-yl group; and R5 is H, alkyl, or aminoalkyl; are useful for modulating a peroxisome proliferator activated receptor, particularly in the treatment of diabetes mellitus.
Owner:ELI LILLY & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds and organic light emitting device containing same 2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds and organic light emitting device containing same](https://images-eureka.patsnap.com/patent_img/78c8cc7d-11d9-423a-aceb-073362f67dfb/HDA0000666937930000011.PNG)
![2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds and organic light emitting device containing same 2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds and organic light emitting device containing same](https://images-eureka.patsnap.com/patent_img/78c8cc7d-11d9-423a-aceb-073362f67dfb/HDA0000666937930000012.PNG)
![2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds and organic light emitting device containing same 2,6,6,8-tetra-substituted-6H-benzo[cd]pyrene compounds and organic light emitting device containing same](https://images-eureka.patsnap.com/patent_img/78c8cc7d-11d9-423a-aceb-073362f67dfb/HDA0000666937930000021.PNG)