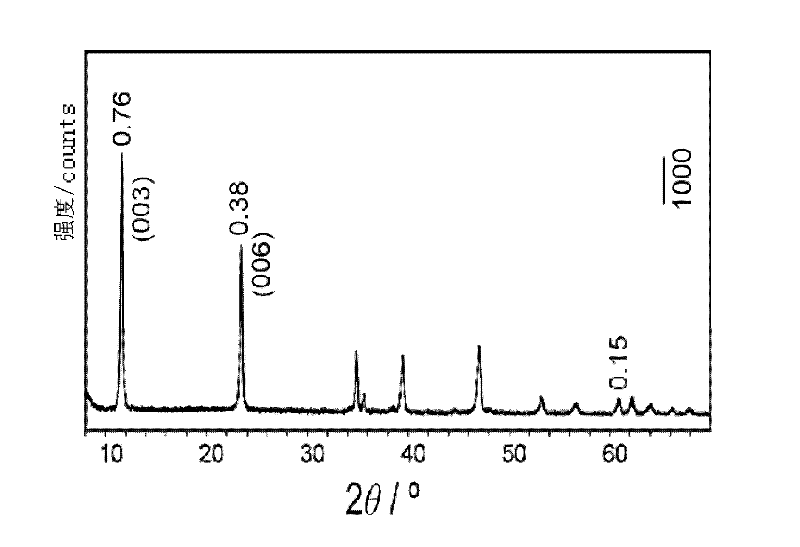
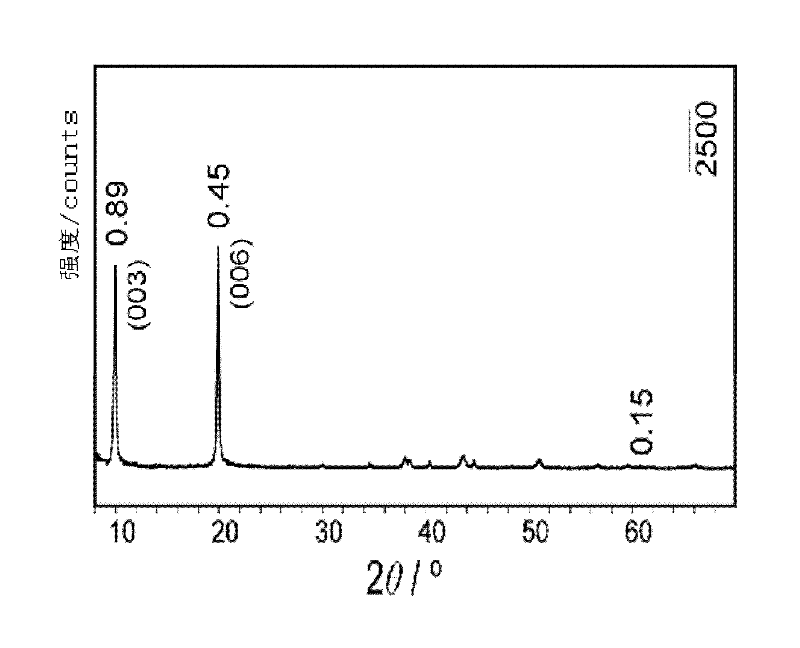
Patents
Literature
100 results about "18-Crown-6" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
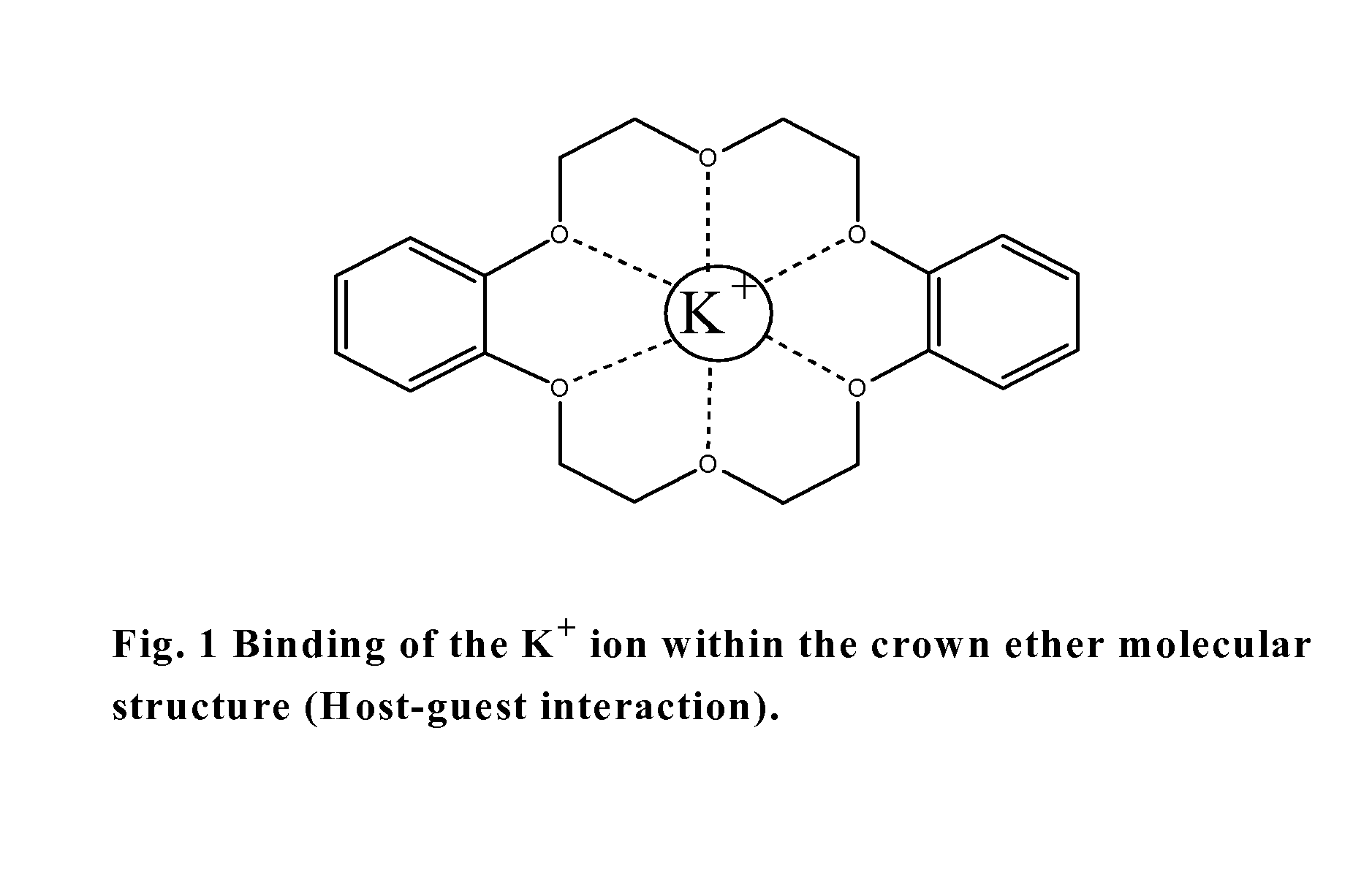
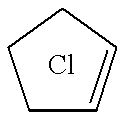
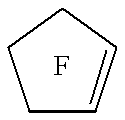
18-Crown-6 is an organic compound with the formula [C₂H₄O]₆ and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 10⁶ M⁻¹). The point group of 18-crown-6 is S₆. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is 2.76 ± 0.06 D in cyclohexane and 2.73 ± 0.02 in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen.
Process for producing fluoroolefins
InactiveCN1589248APreparation by hydrogen halide split-offOrganic chemistry methodsPhosphonium saltPotassium
A process for producing a fluoroolefin of the formula: CF3CY=CXnHp wherein Y is a hydrogen atom or a halogen atom (i.e., fluorine, chlorine, bromine or iodine); X is a hydrogen atom or a halogen atom (i.e., fluorine, chlorine, bromine or iodine); nand p are integers independently equal to 0, 1 or 2, provided that (n + p) = 2; comprising contacting, in the presence of a phase transfer catalyst, a compound of the formula: CF3C (R<l>aR<2>b) C (R<3>CR<4>d) , wherein R<1>, R<2>, R<3>, and R<4> are independently a hydrogen atom or a halogen selected from the group consisting of fluorine, chlorine, bromine and iodine, provided that at least one of R<1>, R<2>, R<3>, and R<4> is halogen and there is at least one hydrogen and one halogen on adjacent carbon atoms; a and b are independently = 0, 1 or 2 and (a+b) = 2; and c and d are independently = 0, 1, 2 or 3 and (c+d) = 3; and at least one alkali metal hydroxide. The alkali metal hydroxide can be, for example, potassium or sodium hydroxide and the phase transfer catalyst can be, for example, at least one: crown ether such as 18-crown-6 and ls-crown-S; or onium salt such as, quaternary phosphonium salt and quaternary ammonium salt. The olefin is useful, for example, as an intermediate for producing other industrial chemicals and as a monomer for producing oligomers and polymers.
Owner:HONEYWELL INT INC
Method of purifying and detecting metal ion in dimethyl sulfoxide
InactiveCN1887864ALow costSimple and fast operationOrganic chemistryOrganic compound preparationIon contentMetal impurities
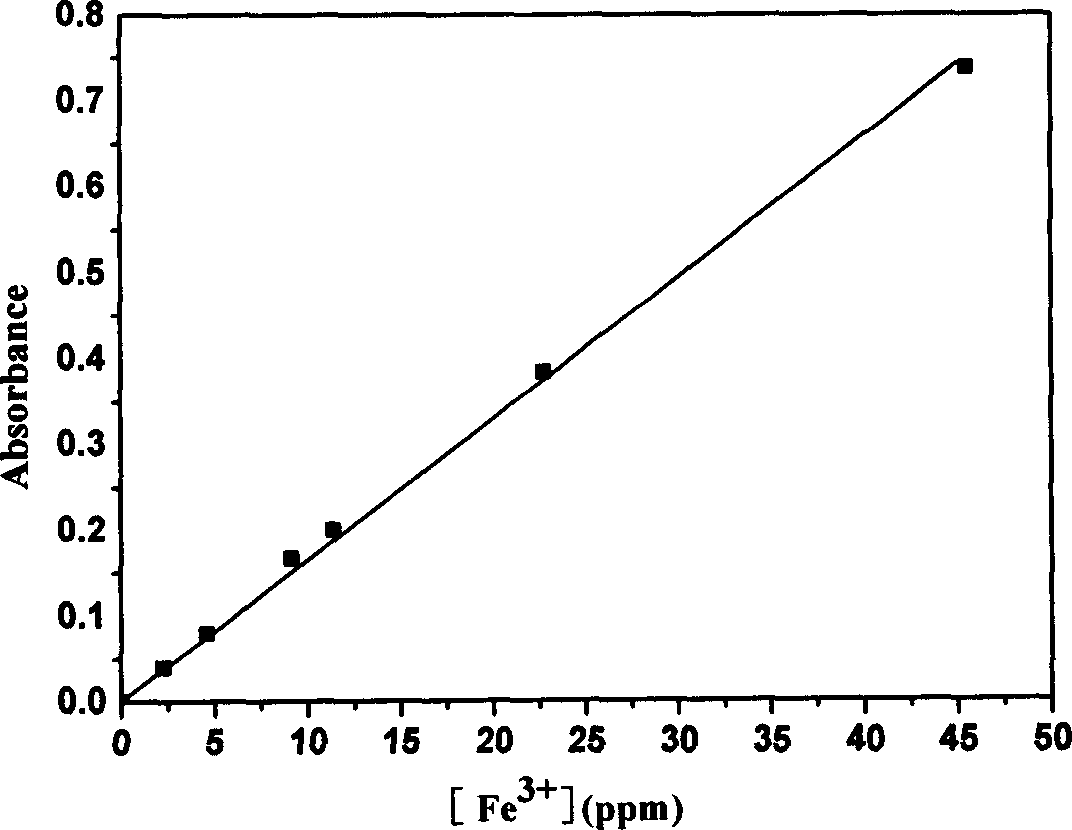
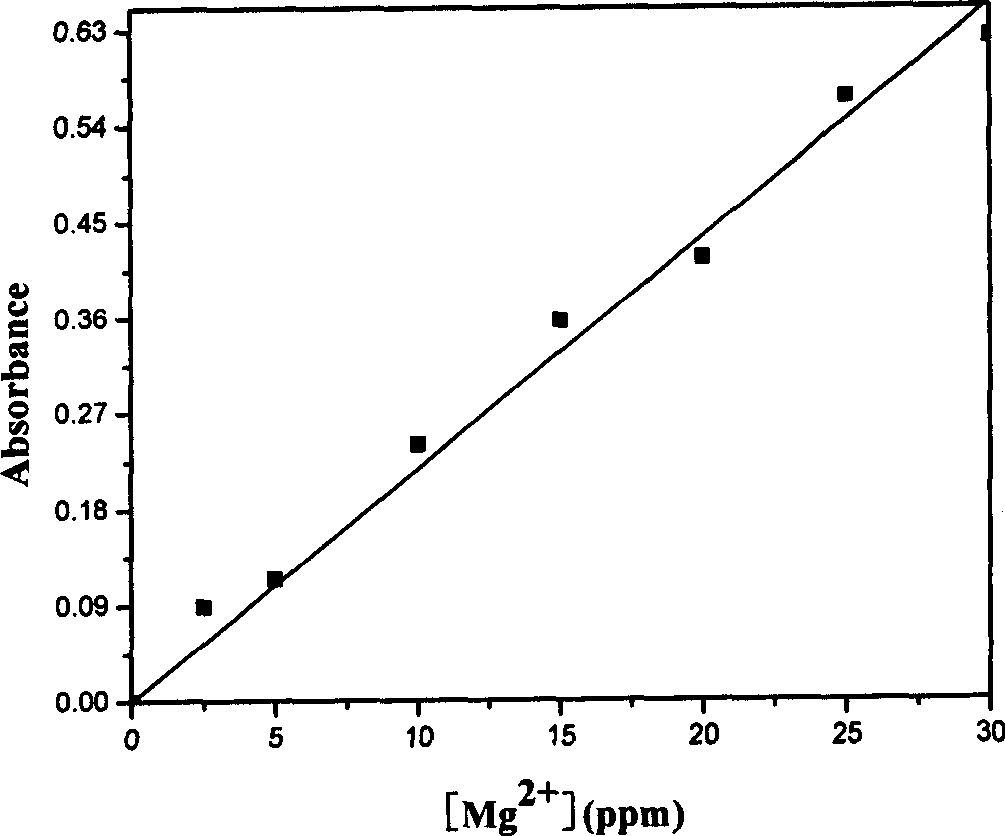
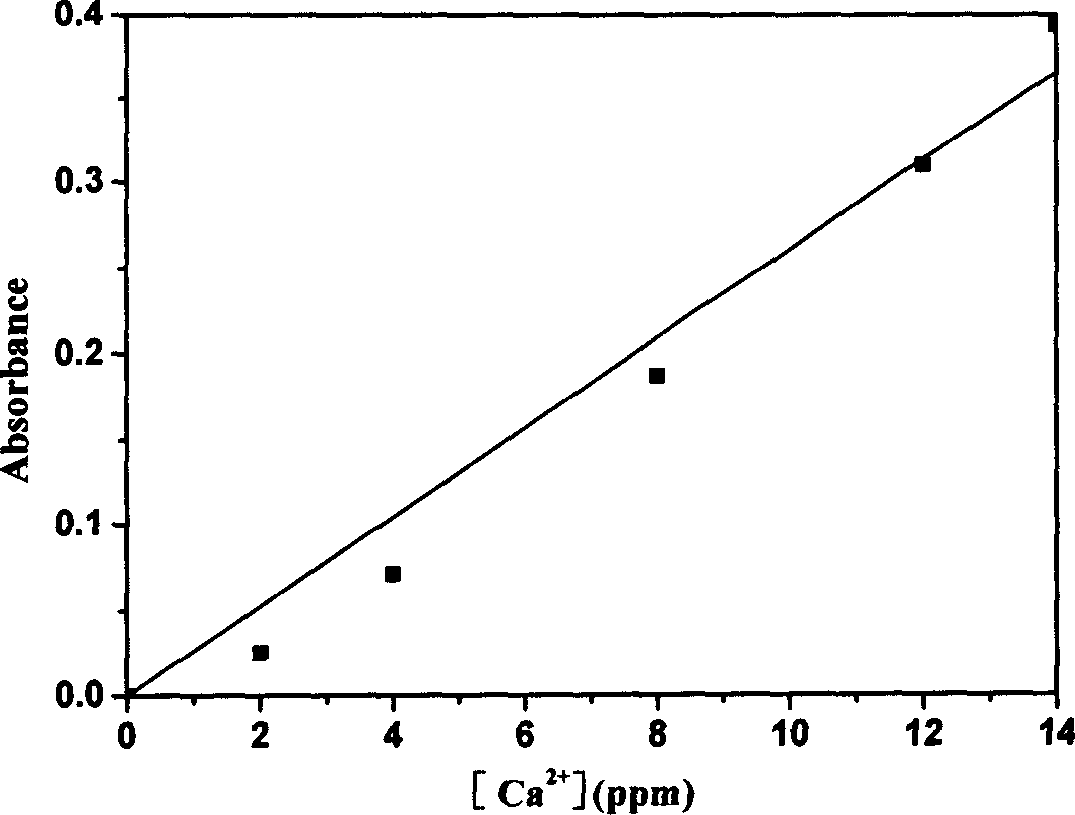
The present invention relates to method of purifying and detecting metal ion in dimetyl sulfoxide as the solution for polymerizing polyactylonitrile carbon fiber. The metal impurities are eliminated from dimetyl sulfoxide based on static adsorption and exchange principle, and the elimination includes eliminating iron ion from dimetyl sulfoxide with macroporous weak alkali type anion exchange resin and eliminating calcium, magnesium, sodium and potassium ions from dimetyl sulfoxide with macroporous strong base type cation exchange resin. The detection includes ultraviolet spectrophotometry to detect the concentrations of various metal ions in the organic solvent, o-phenanthroline colorimetry to detect iron ion content, chloroposphonazo I colorimetry to detect calcium and magnesium ion content, and 18-crown-6 synergistic extraction process to detect potassium ion content.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
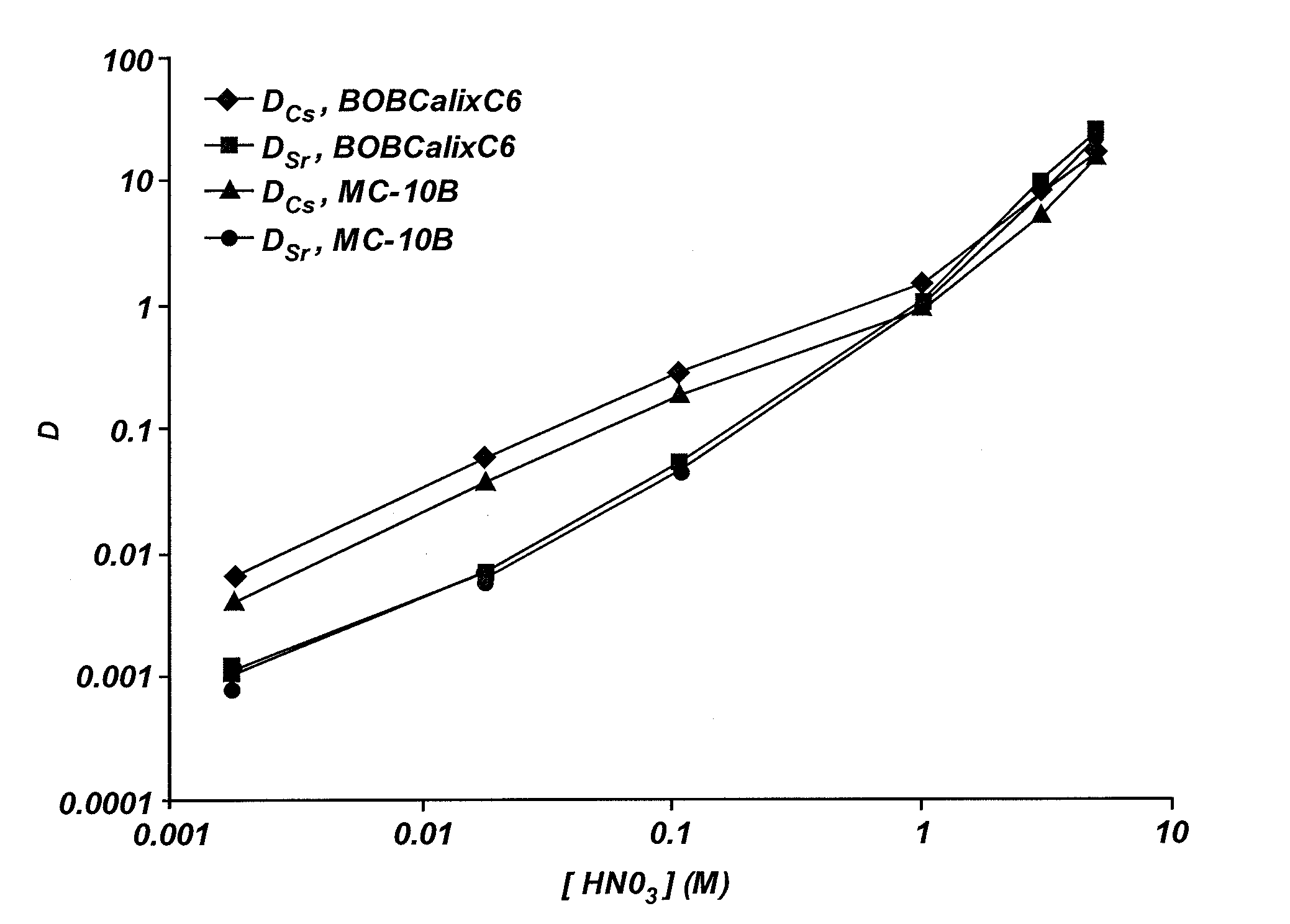
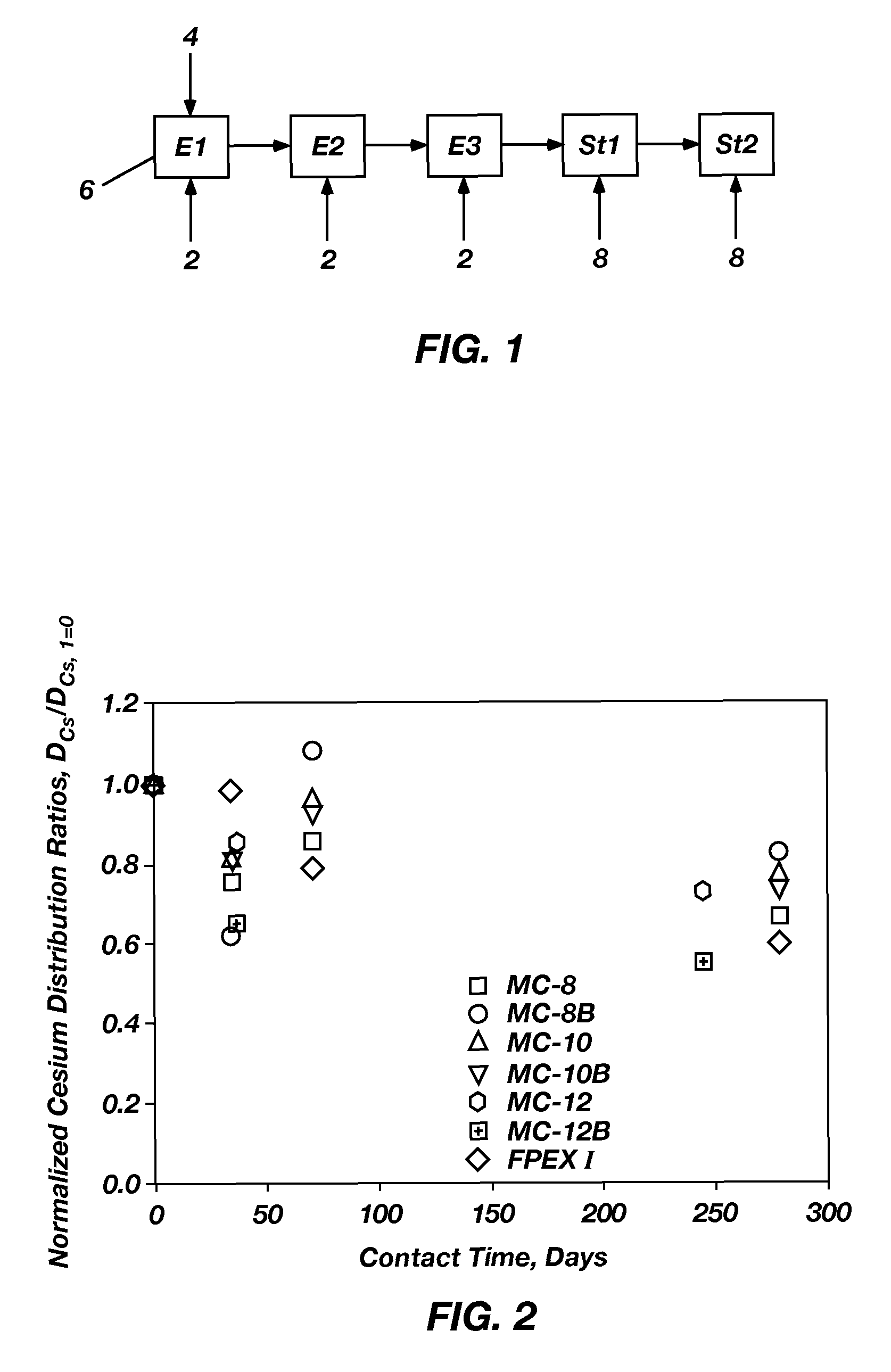
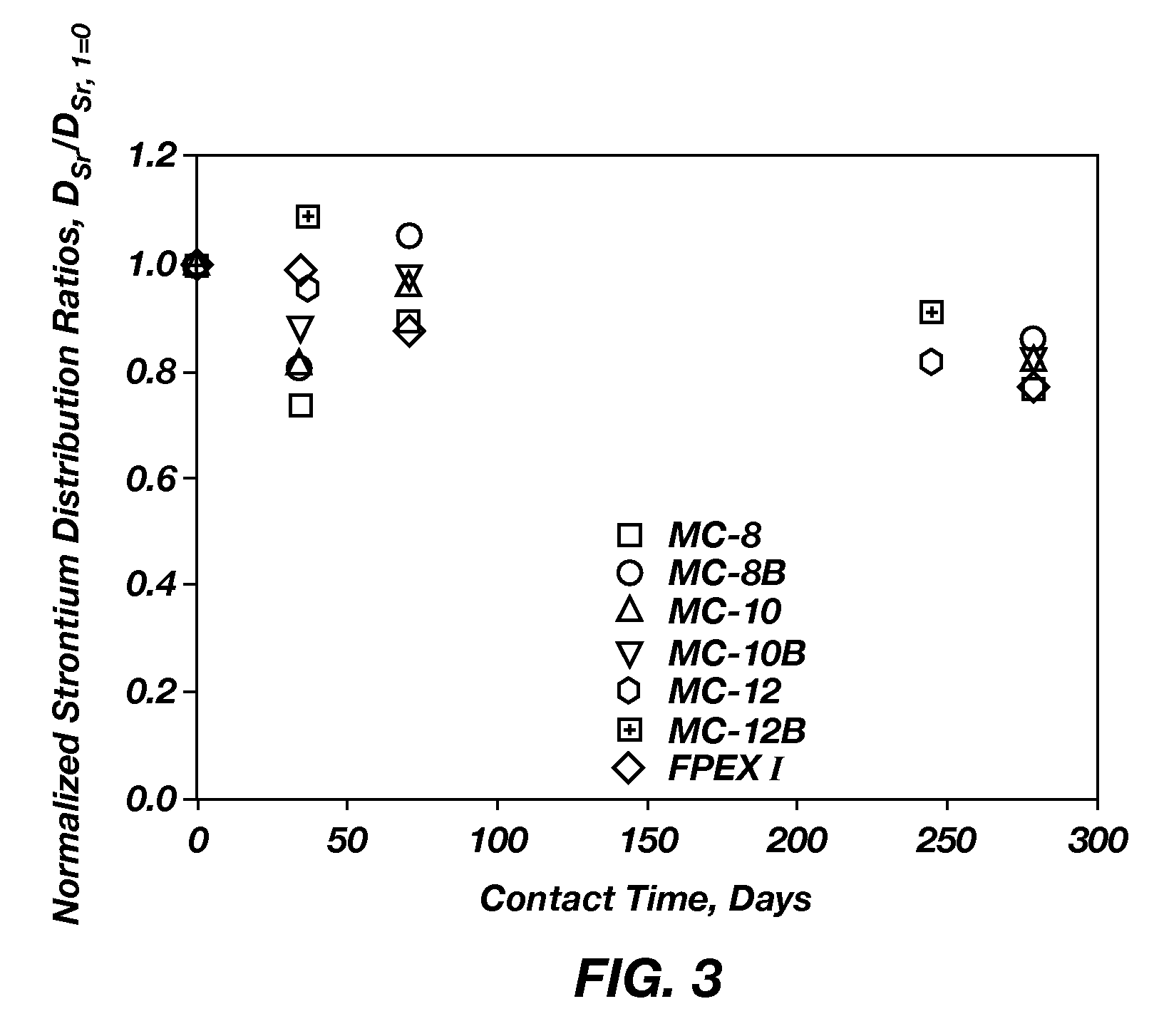
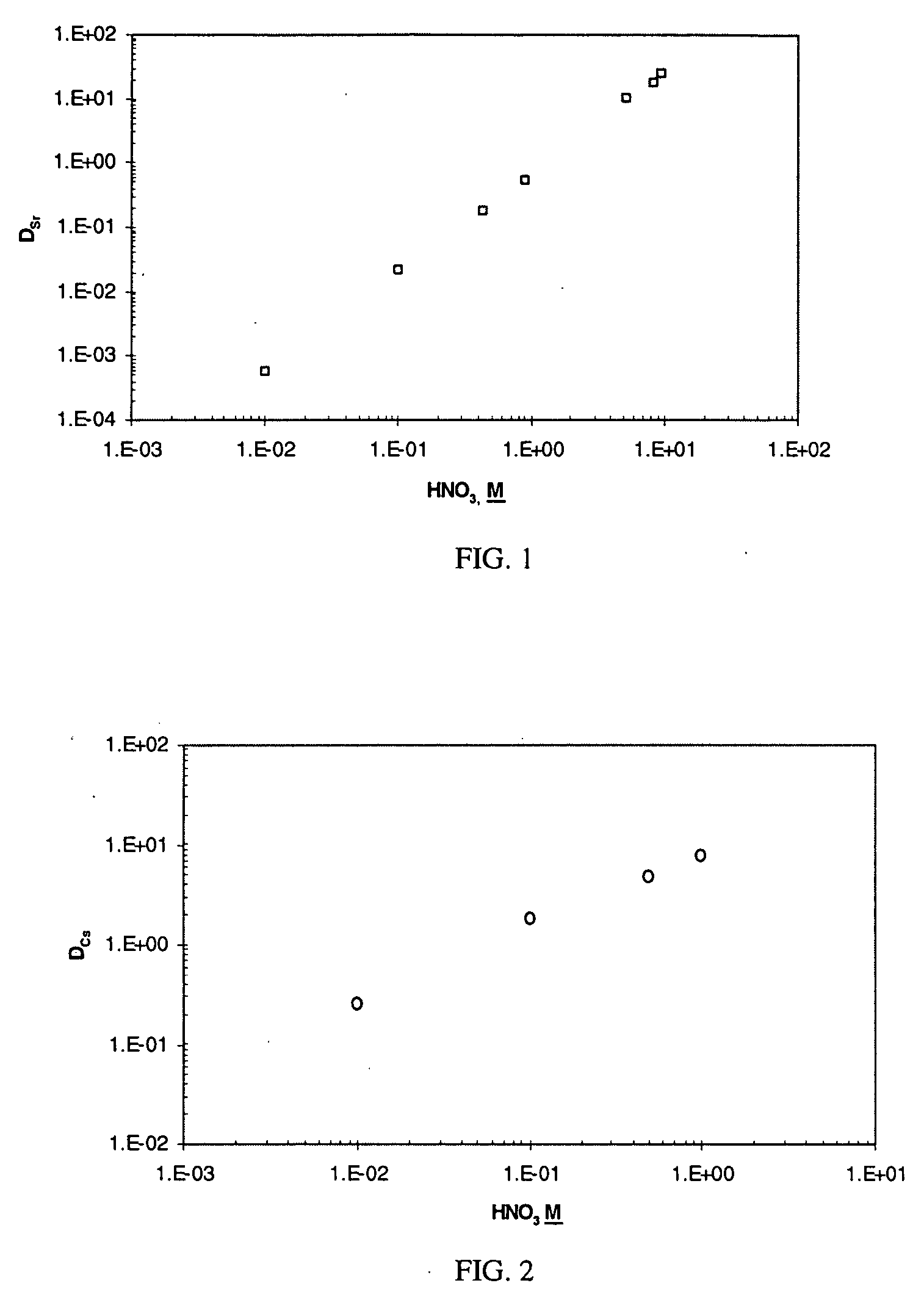
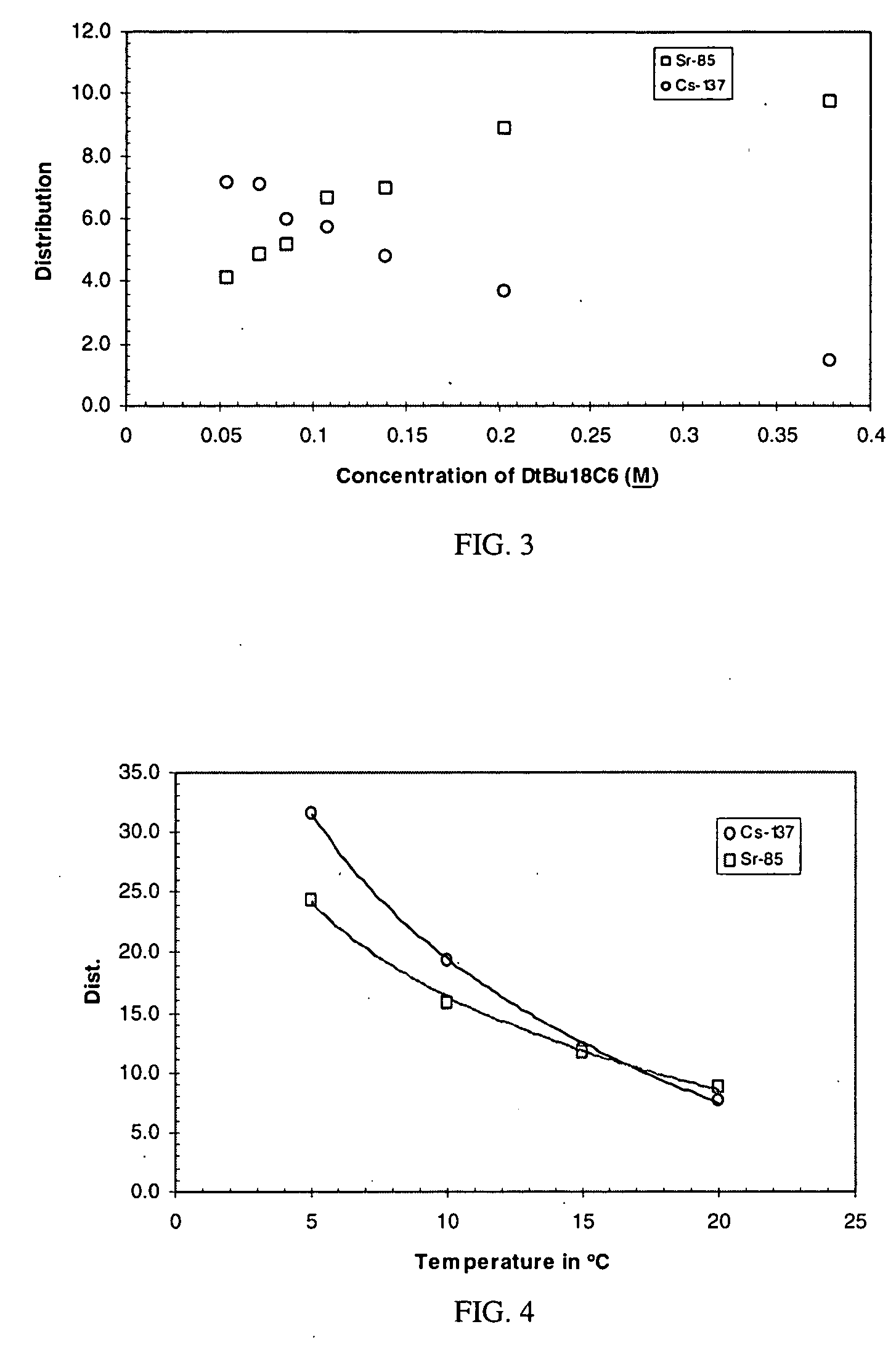
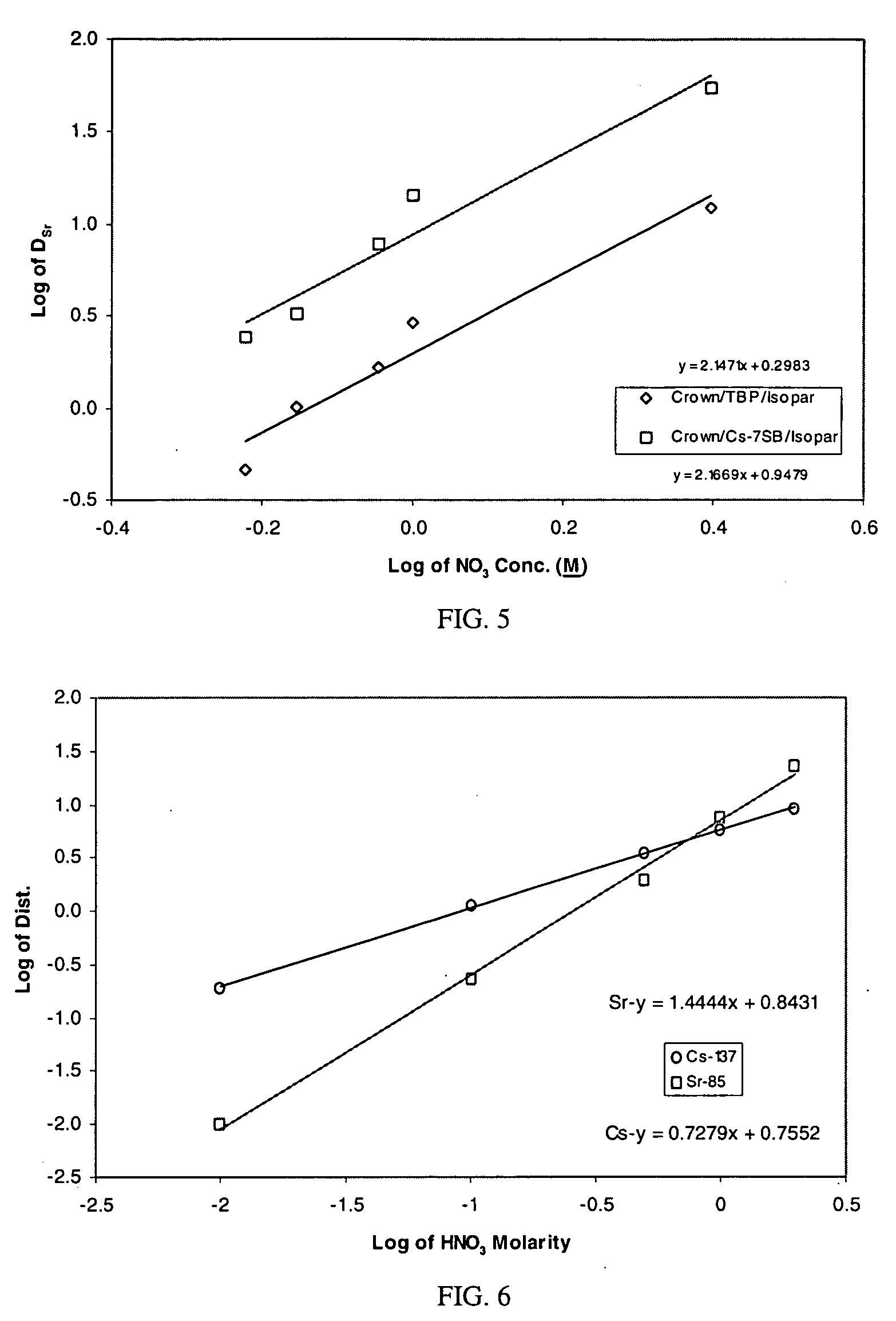
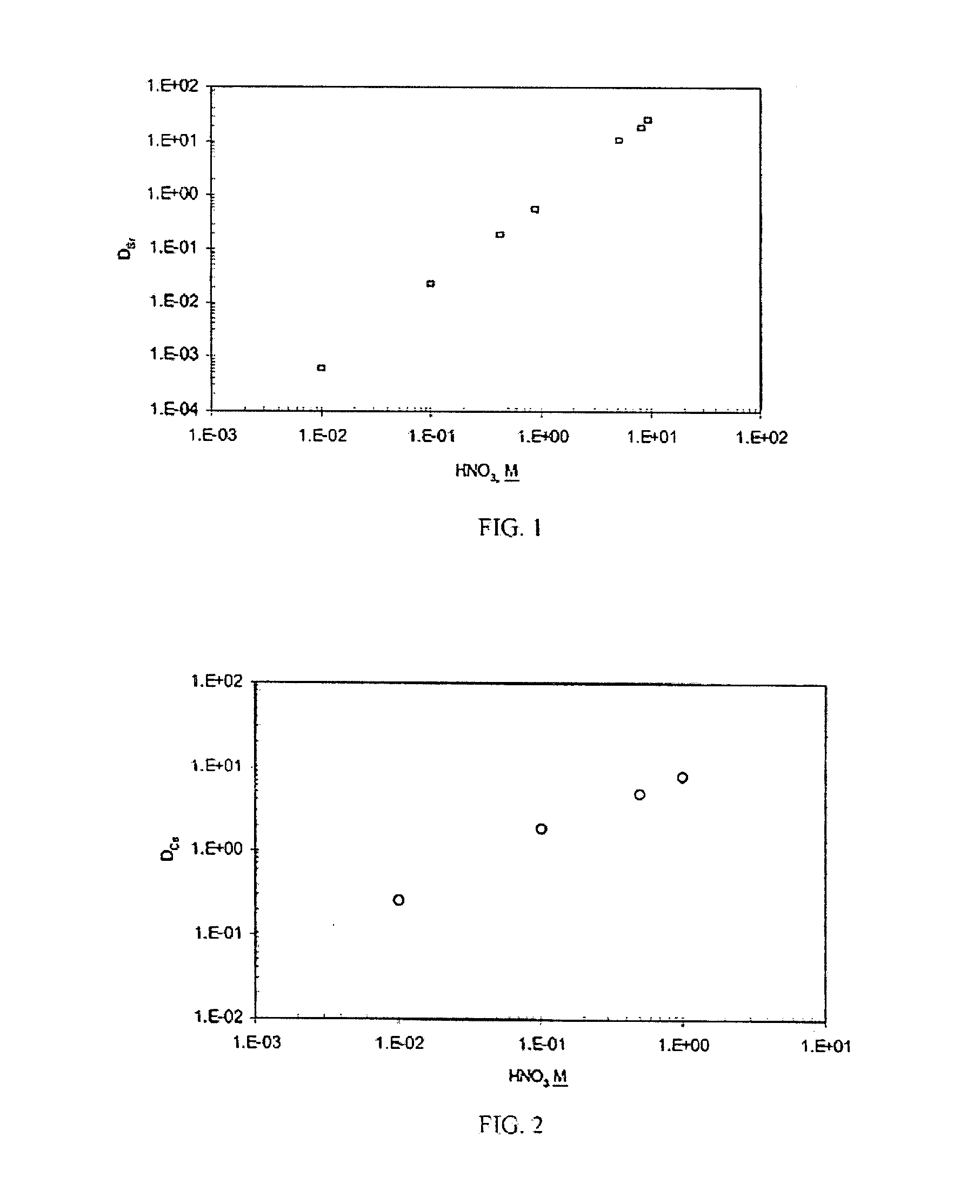
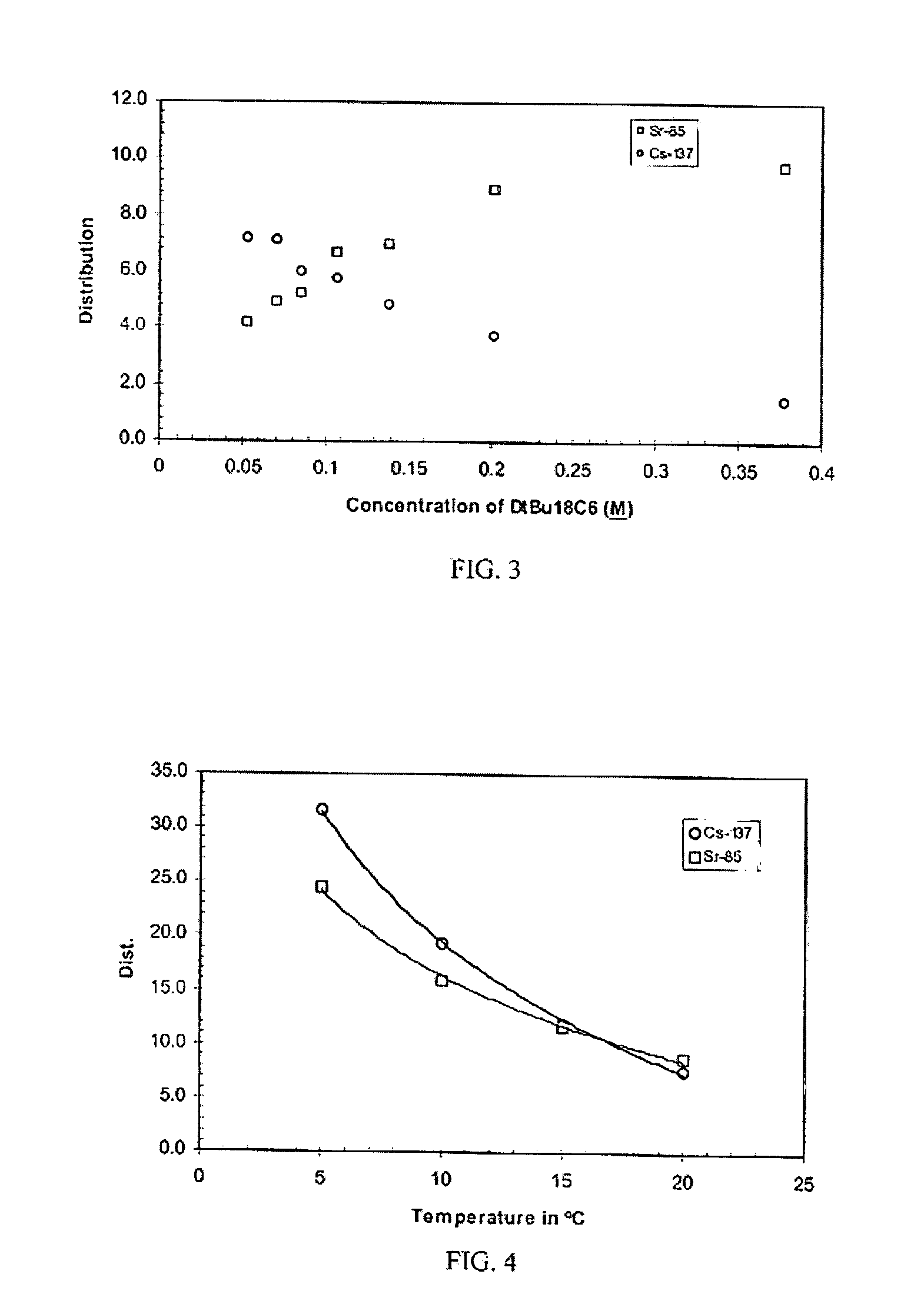
Cesium and strontium extraction using a mixed extractant solvent including crown ether and calixarene extractants
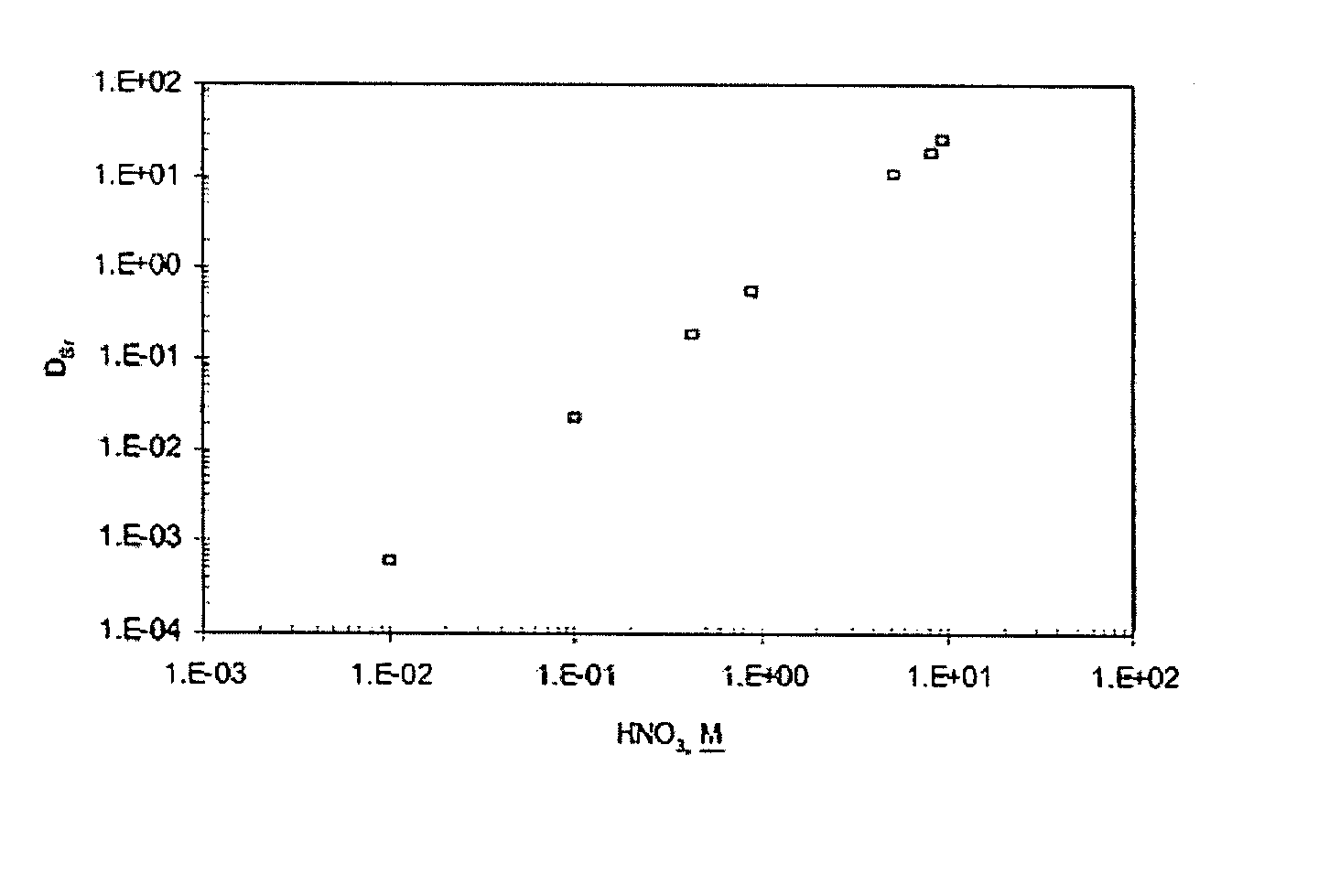
A mixed extractant solvent including calix[4]arene-bis-(tert-octylbenzo)-crown-6 (“BOBCalixC6”), 4′,4′,(5′)-di-(t-butyldicyclo-hexano)-18-crown-6 (“DtBu18C6”), and at least one modifier dissolved in a diluent. The mixed extractant solvent may be used to remove cesium and strontium from an acidic solution. The DtBu18C6 may be present from approximately 0.01 M to approximately 0.4M, such as from approximately 0.086 M to approximately 0.108 M. The modifier may be 1-(2,2,3,3-tetrafluoropropoxy)-3-(4-sec-butylphenoxy)-2-propanol (“Cs-7SB”) and may be present from approximately 0.01M to approximately 0.8M. In one embodiment, the mixed extractant solvent includes approximately 0.15M DtBu18C6, approximately 0.007M BOBCalixC6, and approximately 0.75M Cs-7SB modifier dissolved in an isoparaffinic hydrocarbon diluent. The mixed extractant solvent may form an organic phase in an extraction system that also includes an aqueous phase. Methods of extracting cesium and strontium as well as strontium alone are also disclosed.
Owner:BATTELLE ENERGY ALLIANCE LLC
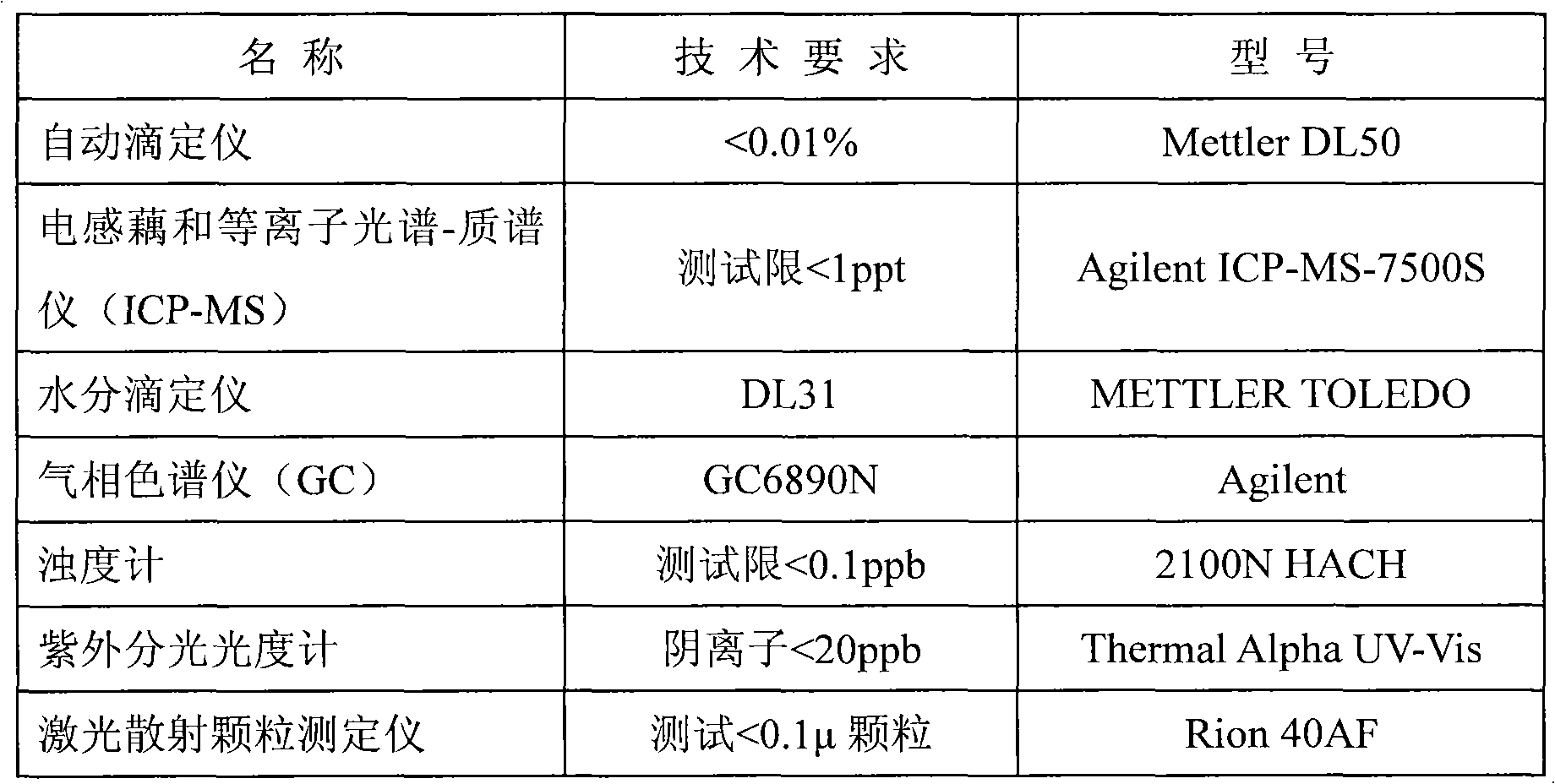
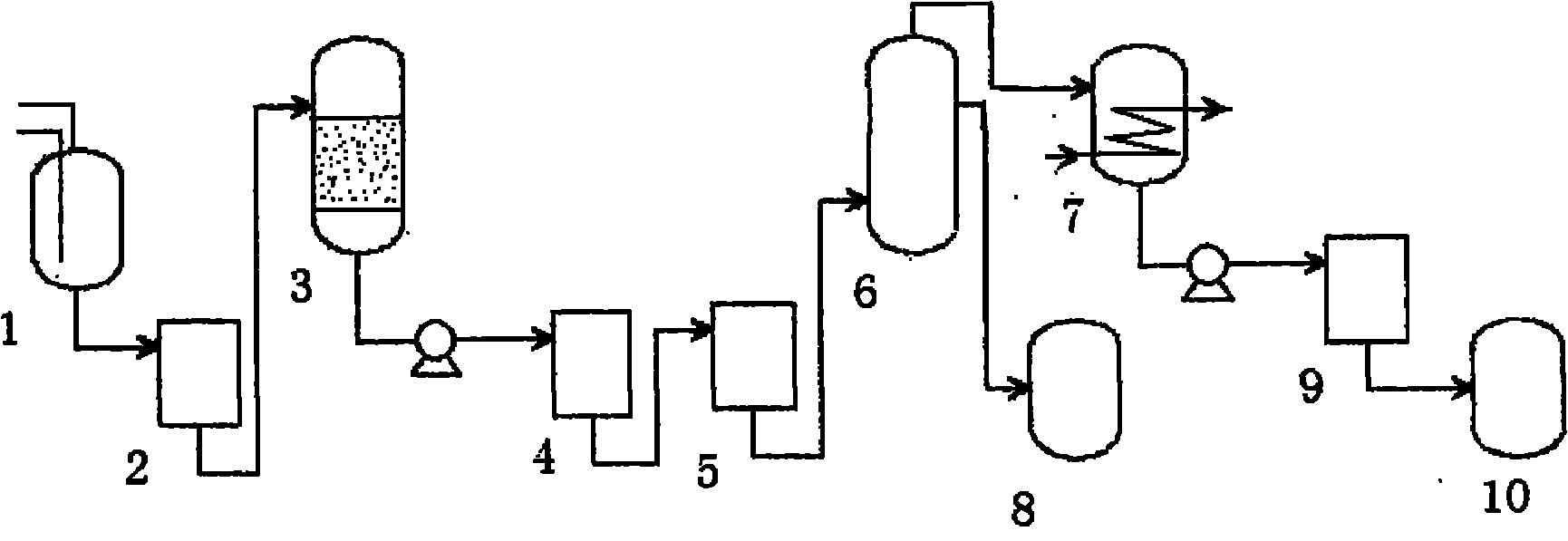
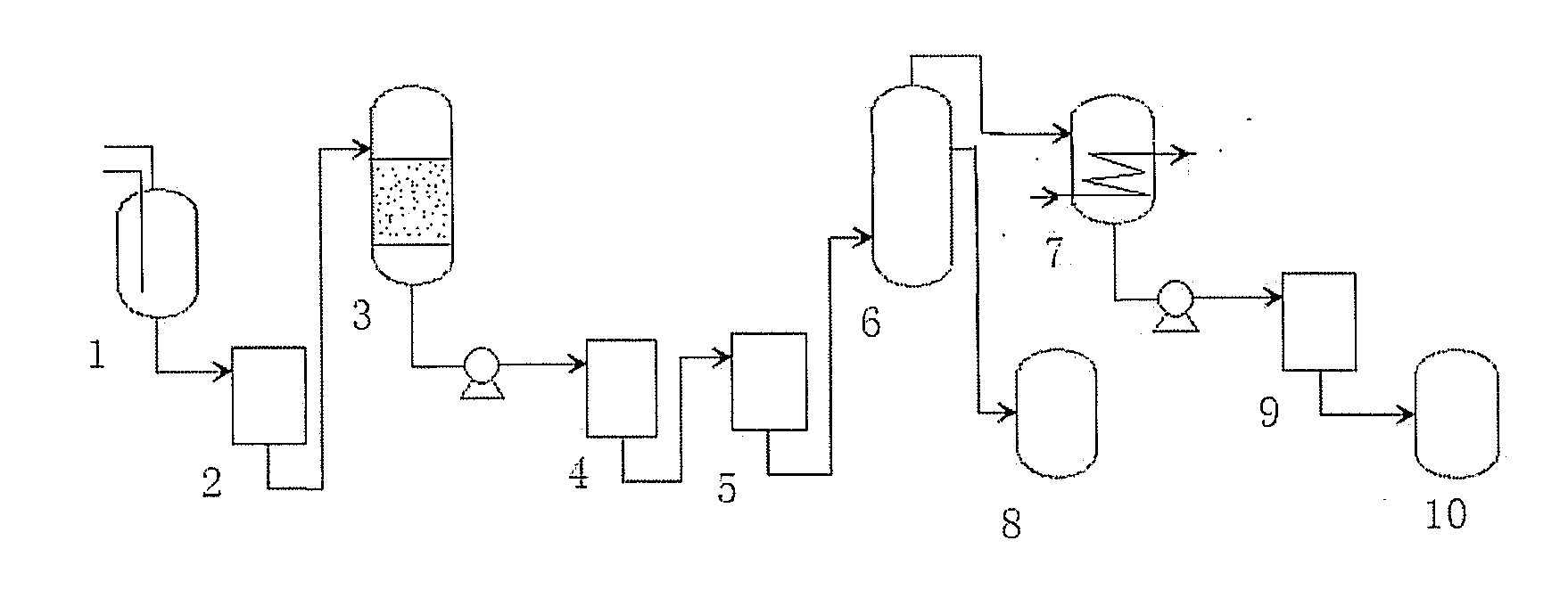
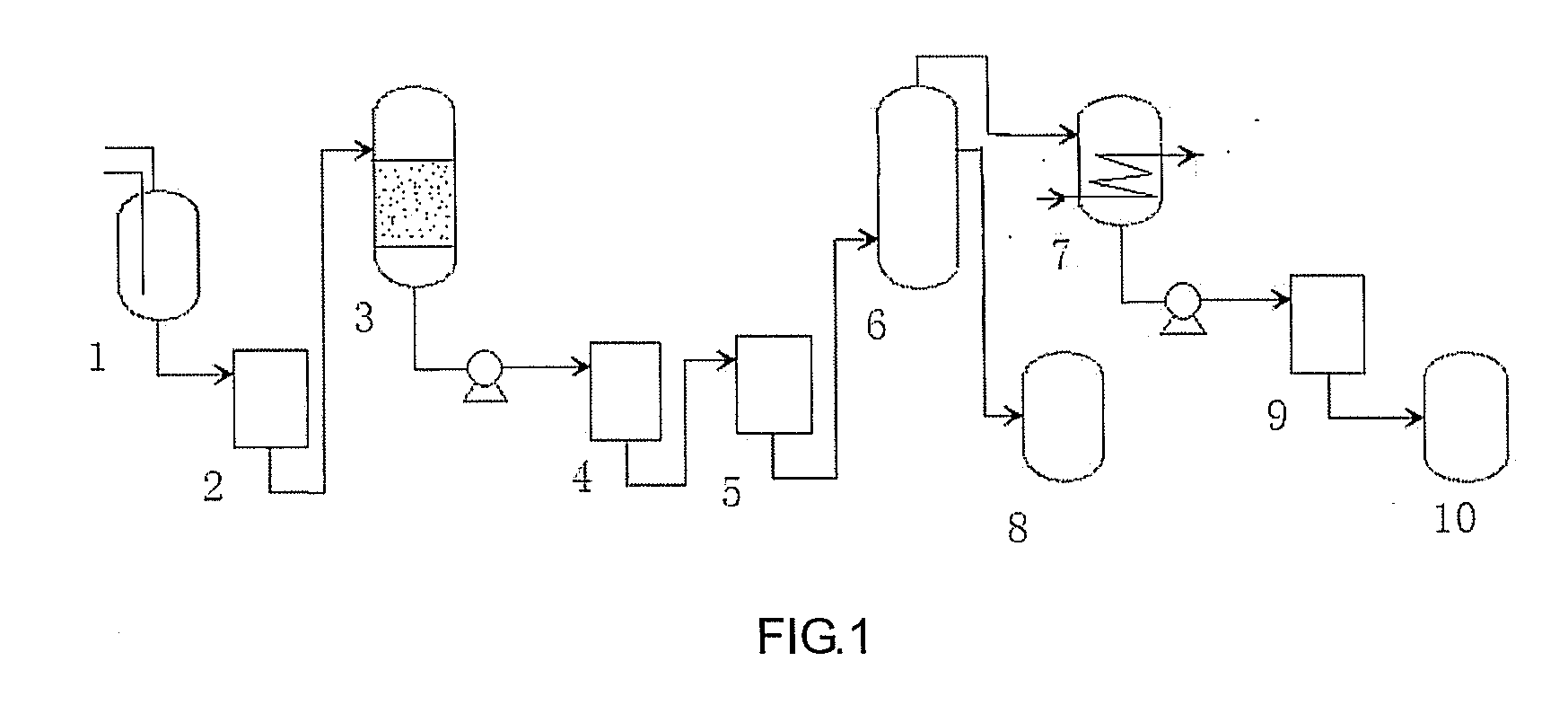
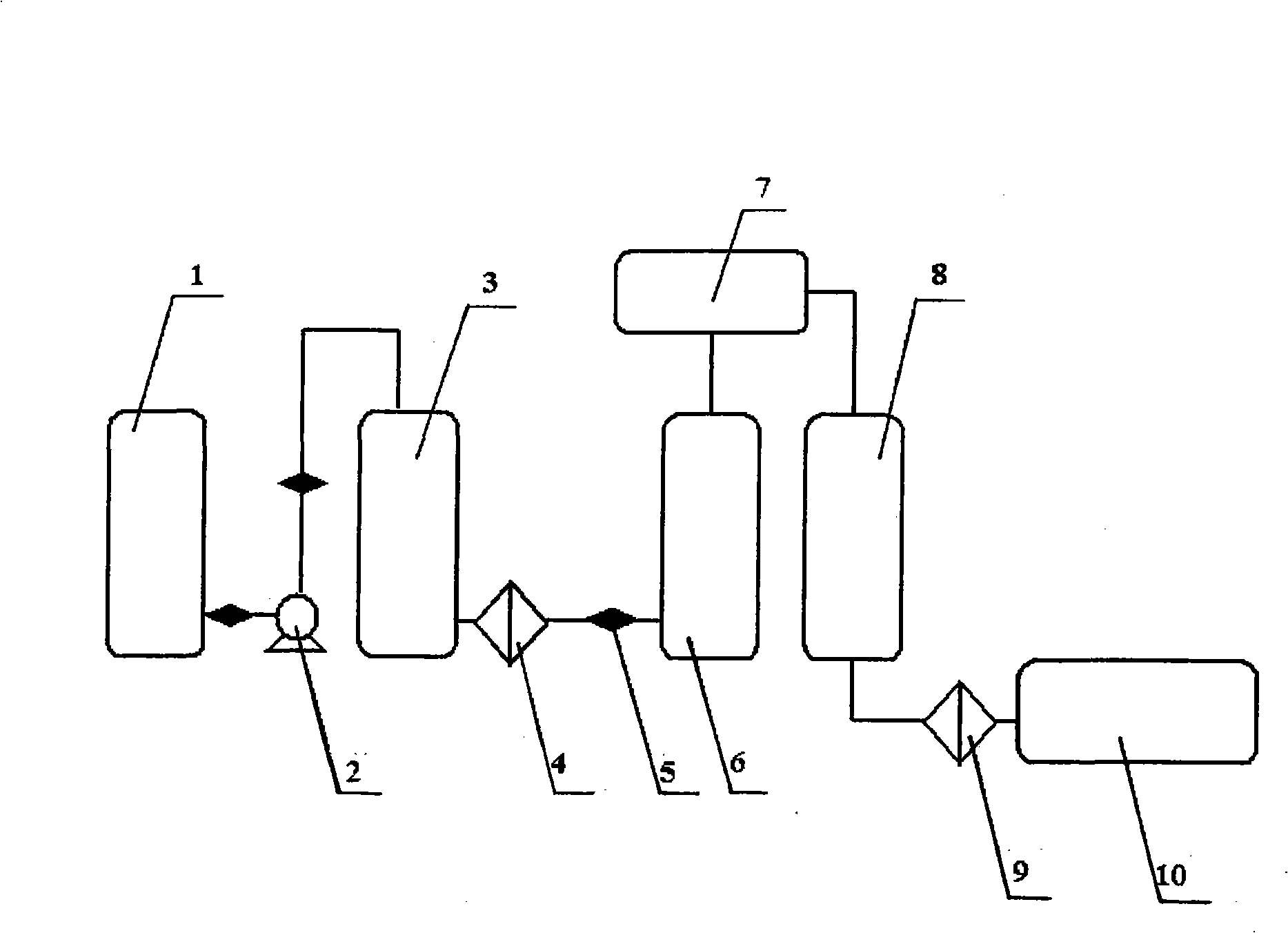
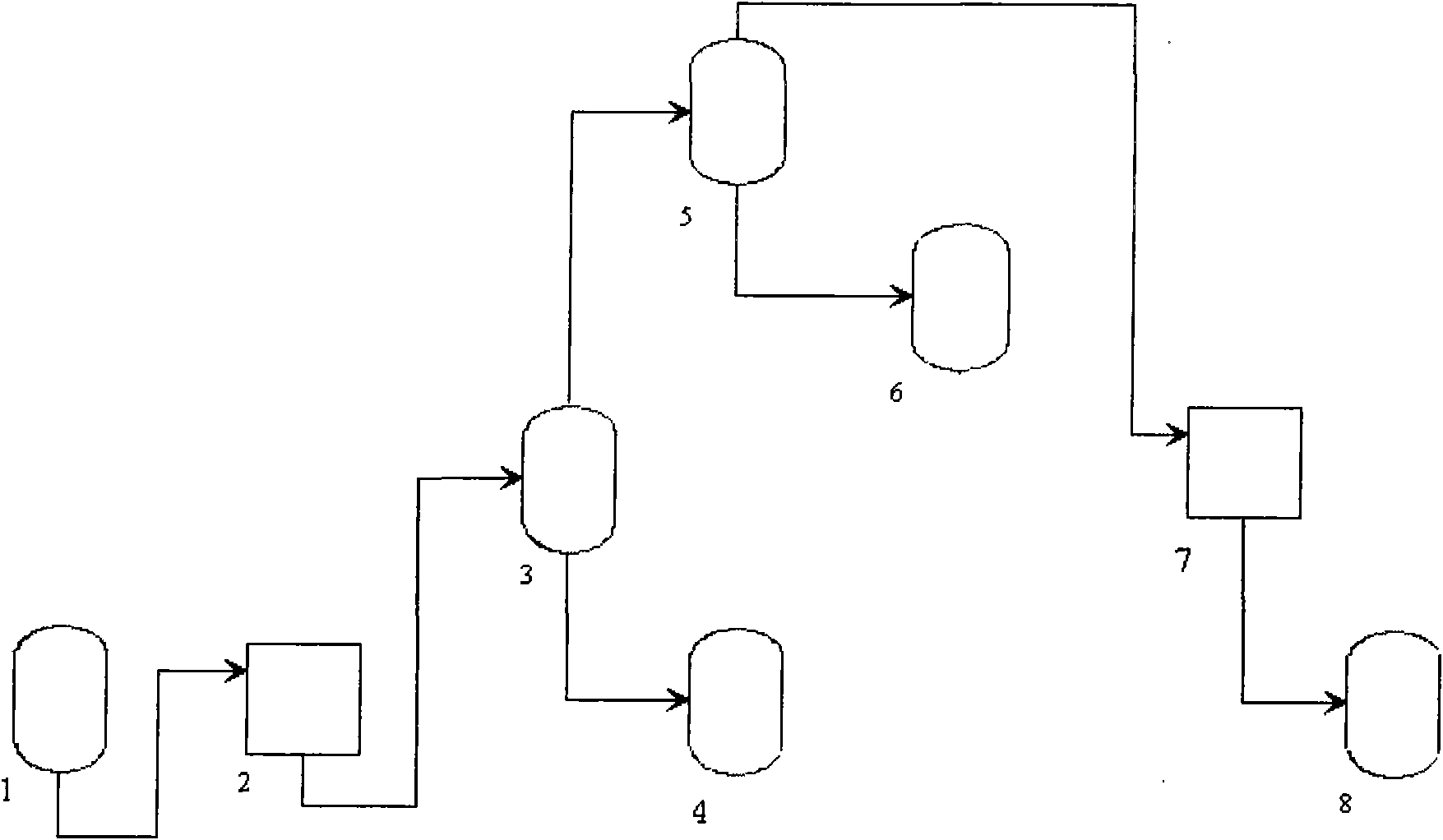
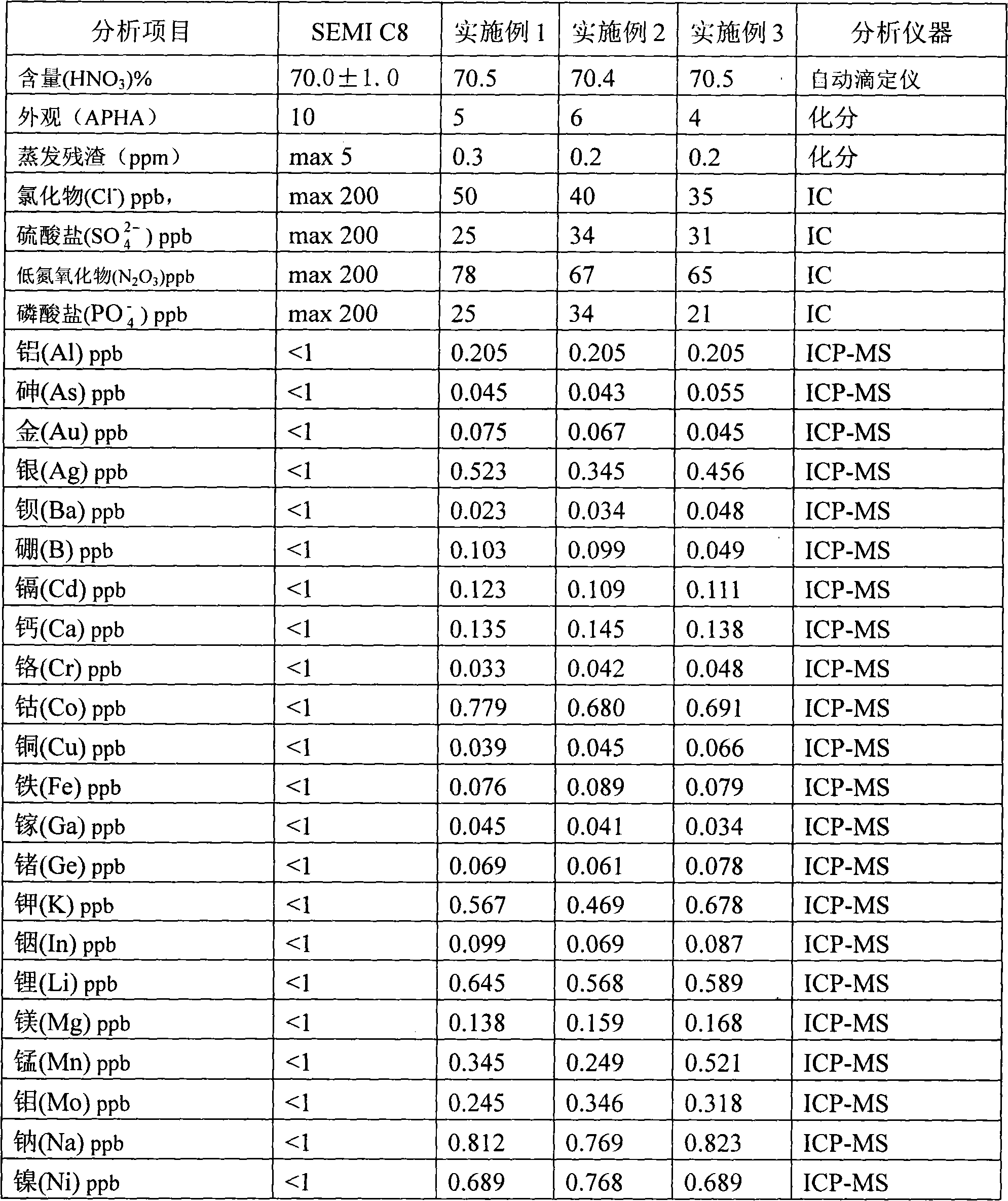
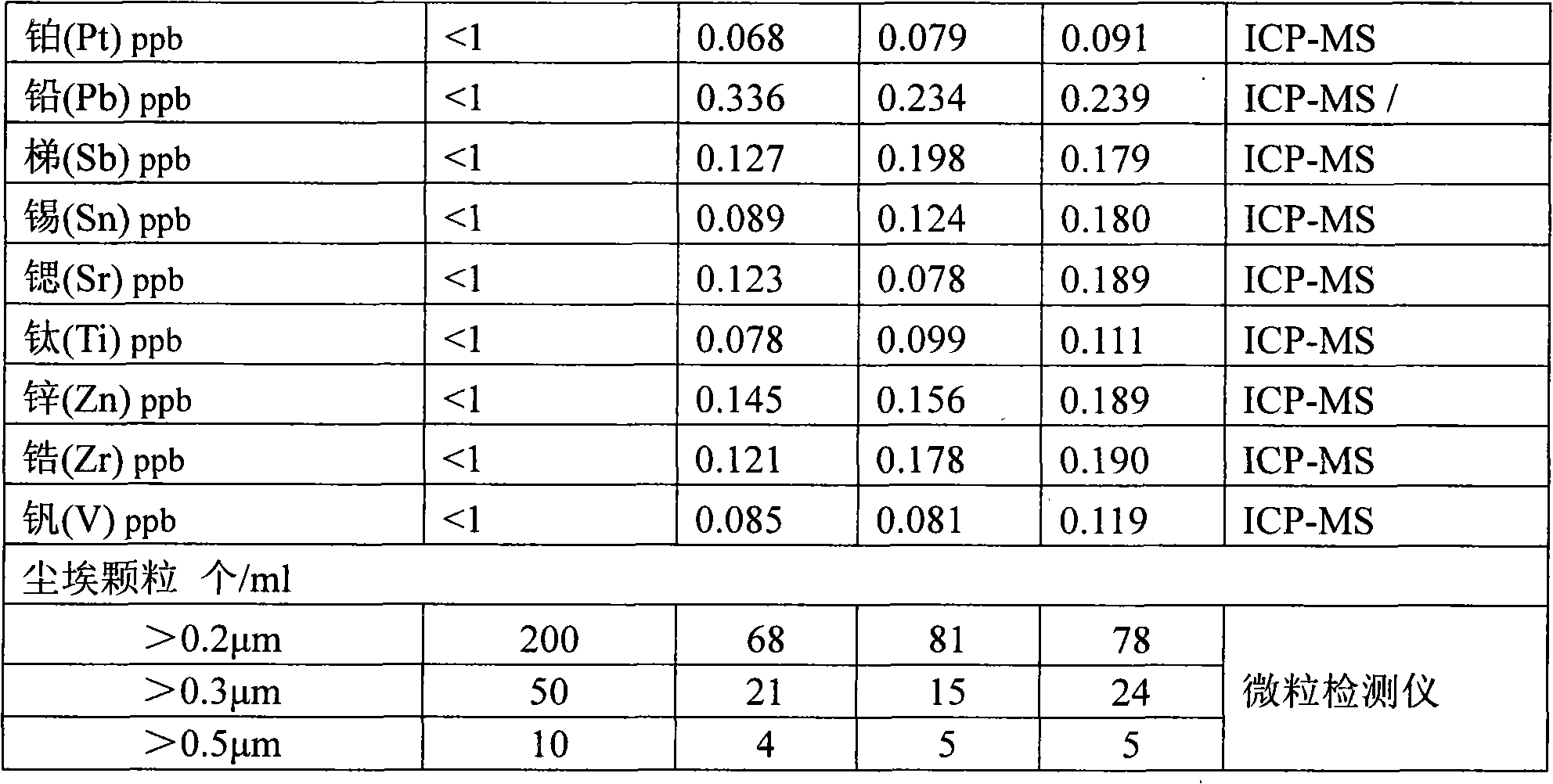
Continuous producing technique for ultra-high pure nitric acid
The invention relates to a process for continuously producing extra high purity nitric acid, which comprises the following steps: first, industrial grade 80-90% nitric acid raw material and double allyl 18-crown-6 ether organosilicon macromolecular complexing agent accounting for 0.2-2% of the weight of nitric acid raw material are mixed in a pretreater, then, the mixture is filtered by a micro-filtration membrane under a working pressure of 0.1-0.2 Mpa, the filtrate enters a rectification tower, and the semi-finished product going through the rectification tower is diluted by ultra pure water in a dilution device; after the dilution is finished, the dissociative NO2 is expelled by high pure nitrogen in a whitening device, and the obtained finished product is filtered by a nanometer filter membrane and enters a finished product receiver under the working pressure of 0.5-0.8 Mpa; wherein, the aperture of the micro filtration membrane is 0.2 to 0.8 Mu m, and the aperture of the nanometer filter membrane is 0.5 to 1.5 nm. In the prepared ultra high purity nitric acid, the content of single cation is lower than 1 ppb, the content of single anion is lower than 100 ppb, and the content of the dust particle larger than 0.5 Mu m is lower than 5 per milliliter. The production process for extra high purity nitric acid has the advantages of simple technique, low production costs, high purity of products, low content of impurity ions, and applicability for large-scale mass production.
Owner:JIANGYIN RUNMA ELECTRONICS MATERIAL
Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide
The invention provides a preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C]pyrimidine-2-)benzsulfamide, comprising the following steps: using a compound represented by formula (IV) as an initial raw material to synthesize a compound represented by formula (III); synthesizing the compound represented by formula (II); finally synthesizing a target compound represented by formula (I). In the invention, two phenolic alcohols are jointed to form ether, thereby preventing from using costly non-marketization 2,2-difluorobromoethane for introducing difluoroethoxy as a reagent; in the process of synthesizing target product, besides 3,5-dimethyl pyridine and dimethyl sulfoxide, a catalytic amount of 18-crown-6 crown ether is used to improve the reaction timeand reaction yield. The invention simplifies the reaction conditions, optimizes the synthesis process, enhances the reaction yield, and reduces production cost, thereby greatly improving the synthesis effect. The reaction formula is represented as follows.
Owner:孙智华 +2
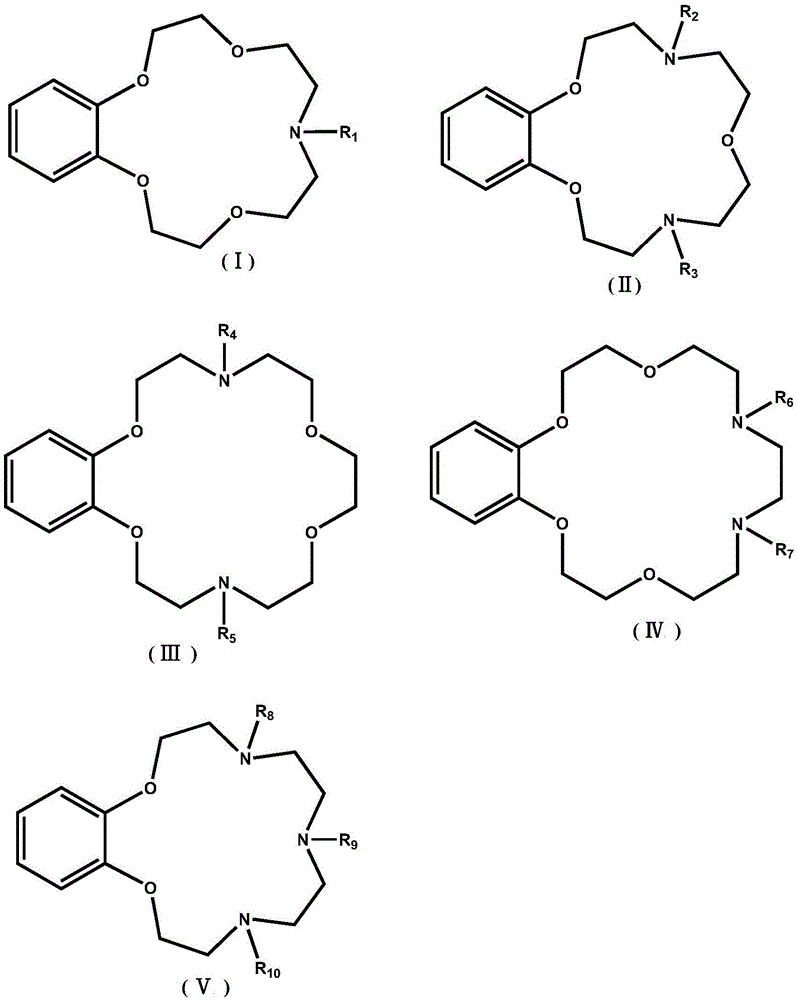
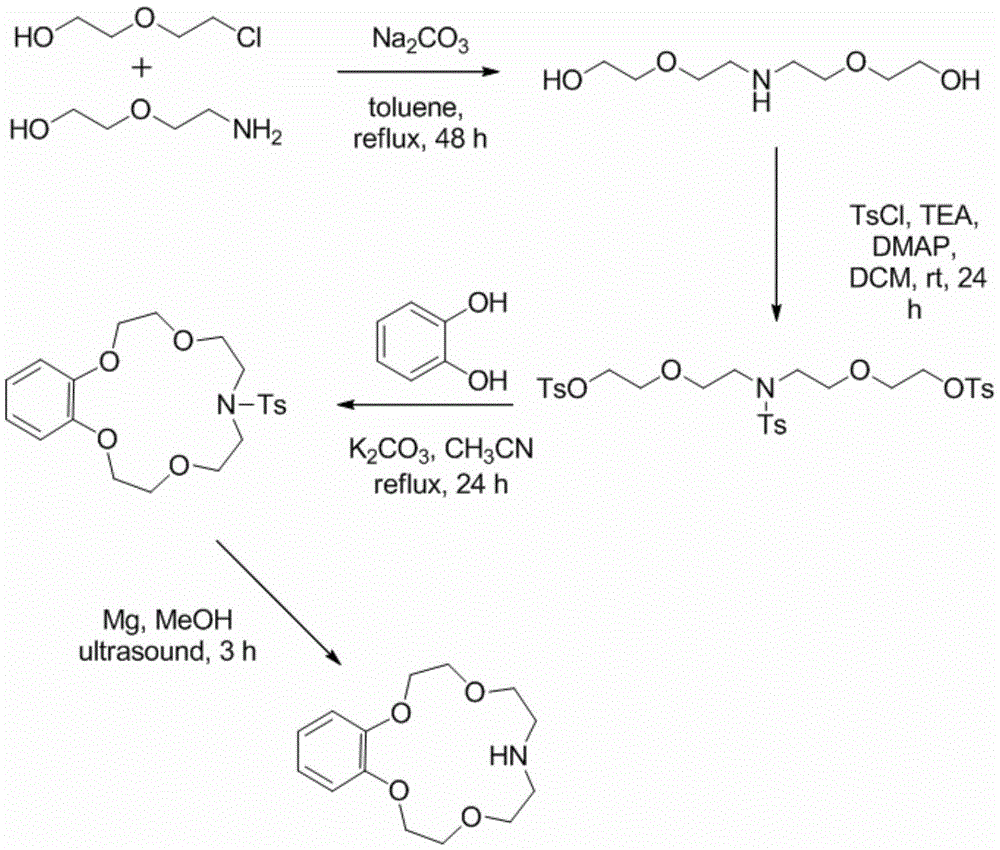
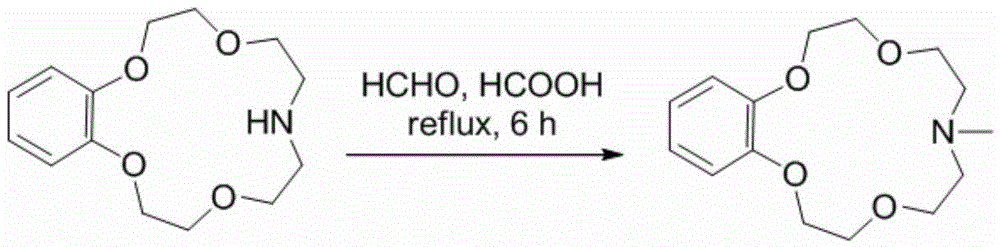
Application of benzo-azacrown ether compounds to separation of lithium isotopes
ActiveCN105561790AEfficient separationFast exchange rateOrganic chemistryIsotope separationIsotope18-Crown-6
The invention discloses application of benzo-azacrown ether compounds to separation of lithium isotopes. The benzo-azacrown ether compounds are selected from monoaza15-crown-5, bisaza15-crown-5, triaza15-crown-5 and bisaza18-crown-6. The benzo-azacrown ether compounds serving as extracting agents are dissolved in organic solvents to prepare organic phases, a lithium trifluoroacetate aqueous solution serves as an aqueous phase, and the lithium isotopes are separated at the room temperature by liquid-liquid extraction. The benzo-azacrown ether compounds are easy to dissolve in the organic solvents, efficient separation of the lithium isotopes can be realized by means of liquid-liquid extraction, and considerable separation factors, simplicity and convenience in operation, quickness in isotope exchange and technical simplicity are realized.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
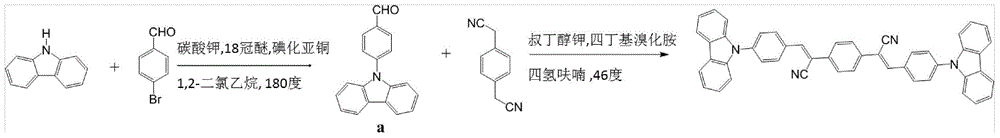
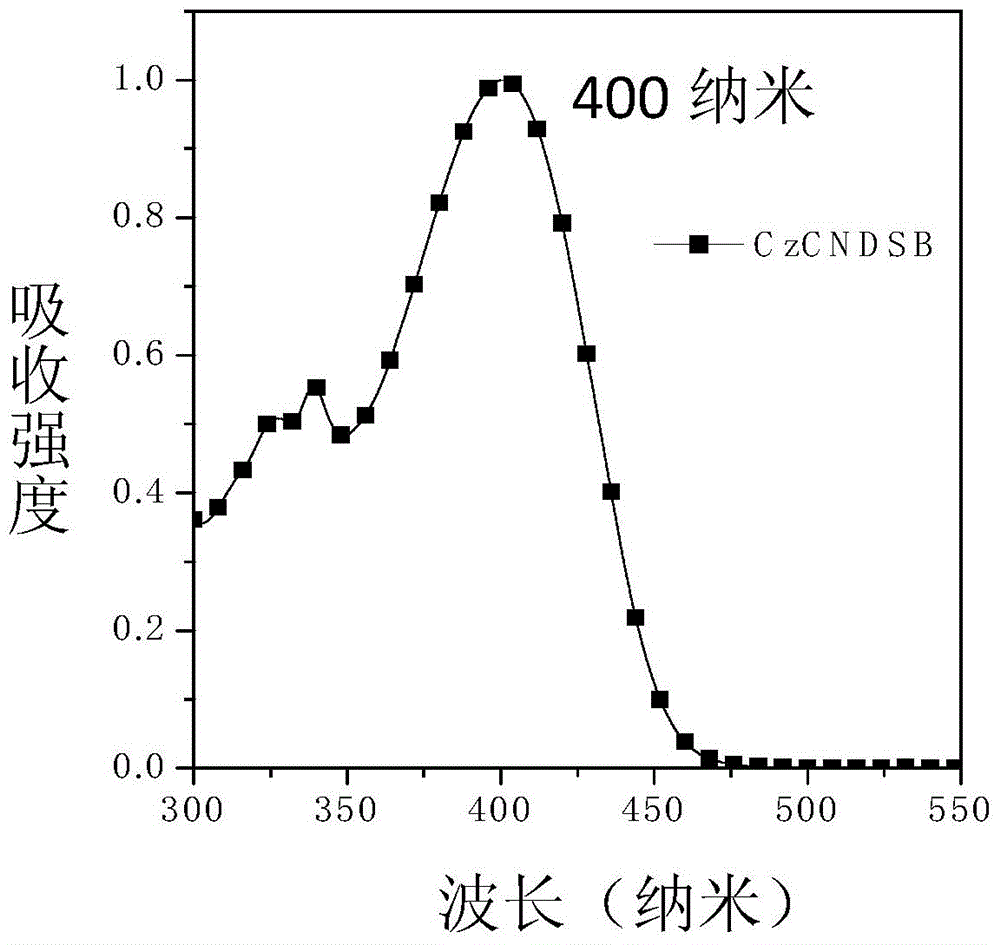
Piezochromic material, preparation method and applications thereof
ActiveCN104312576AImprove luminous efficiencyGood linear relationshipOrganic chemistryTenebresent compositionsIodideEvaporation
The invention discloses a piezochromic material, a preparation method and applications thereof. The preparation method comprises the following steps: adding 3 grams of carbazole, 3.3 grams of p-bromobenzaldehyde, 3.7 grams of potassium carbonate, 0.33 gram of 18-crown-6, 0.4 gram of cuprous iodide, and 18 mL of o-dichlorobenzene into a round-bottom flask (100 mL); vacuumizing under freezing, introducing nitrogen into the round-bottom flask for three times, stirring at a temperature of 180 DEG C, carrying out reflux for 48 hours; repeatedly washing the reaction system by a hydrochloric acid solution (5%), using dichloromethane to carry out extraction for three times, using waterless magnesium sulfate to dry the product, performing rotary evaporation to remove the solvent, and finally carrying out column chromatography isolation to obtain 3.9 grams of pure product, wherein the yield is 80%.
Owner:JILIN UNIV
Photosensitive chiral macrocyclic molecule and preparation method and application thereof
ActiveCN104496933AAchieve complexationAchieve releaseLiquid crystal compositionsOrganic chemistryN dimethylformamideCis trans isomerization
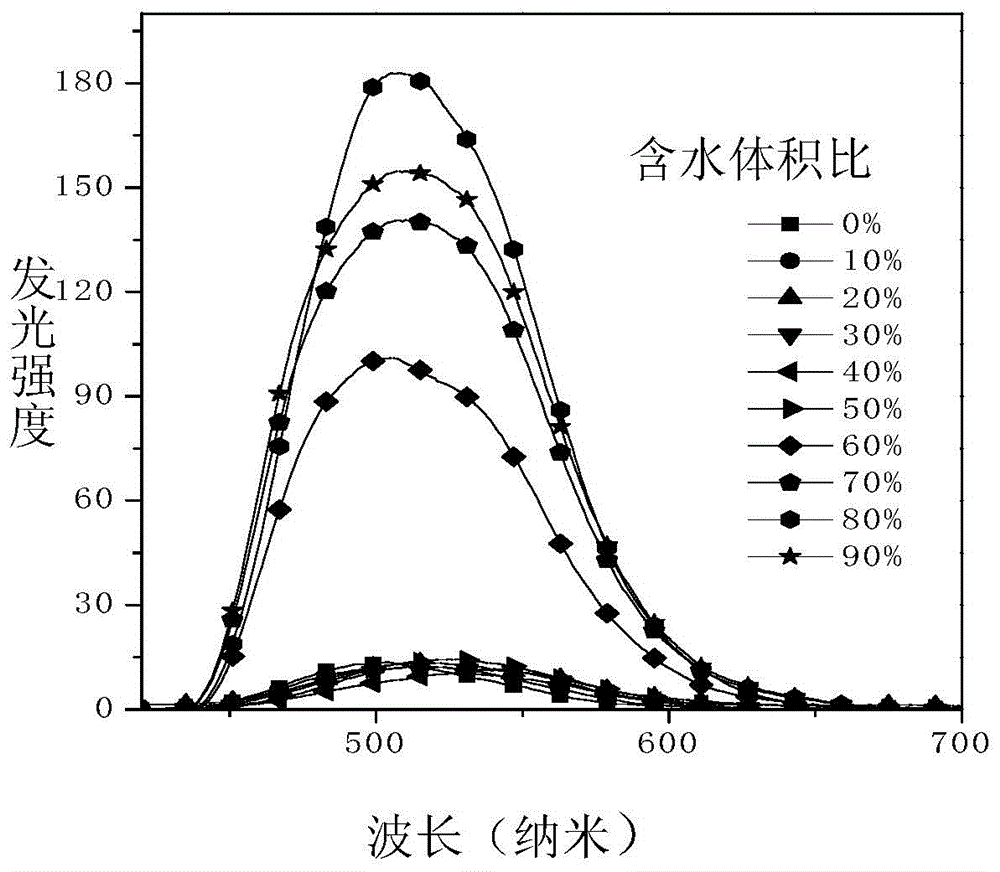
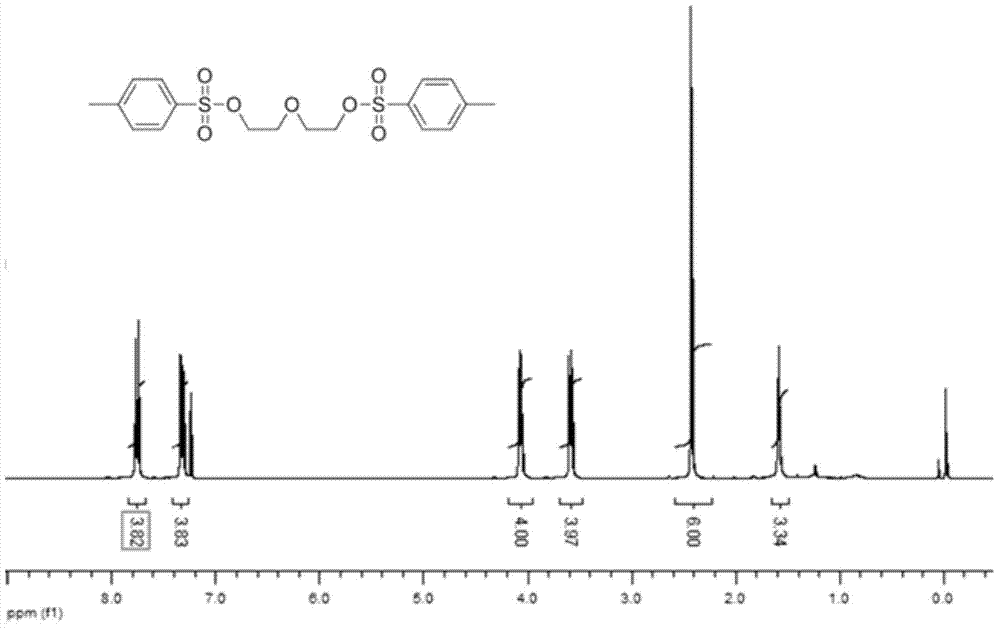
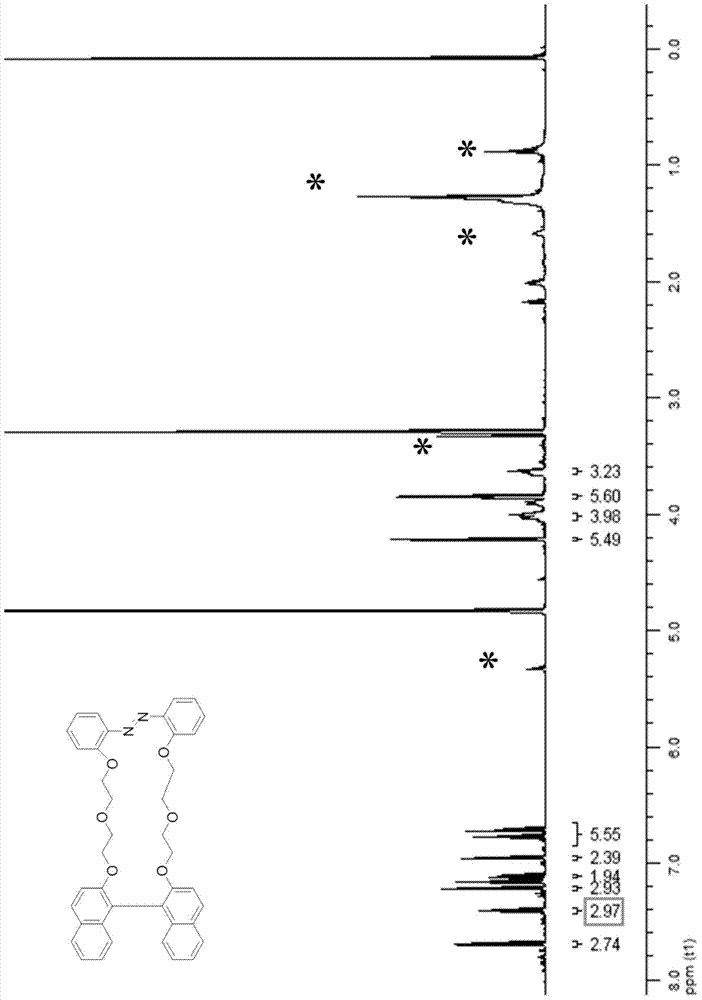
The invention discloses a photosensitive chiral macrocyclic molecule and a preparation method and application thereof, belonging to the field of preparation of photoresponsive materials. The preparation method for the photosensitive chiral macrocyclic molecule adopts the following steps: a) reacting oligomeric ethylene glycol, sodium hydroxide and p-toluenesulfonyl chloride in a tetrahydrofuran solvent in an inert atmosphere so as to obtain an intermediate 1; b) dissolving the intermediate 1, cesium carbonate and dibenzo-18-crown-6 in an N-N dimethylformamide solvent and carrying out a reaction in an inert atmosphere so as to obtain an intermediate 2; and c) dissolving the intermediate 2, chiral binaphthol compounds, cesium carbonate and dibenzo-18-crown-6 in the N-N dimethylformamide solvent and carrying out a reaction in an inert atmosphere so as to obtain a target product. The photosensitive chiral macrocyclic molecule provided by the invention has the advantages of easy synthesis, good stability, ability of selective complexation of chiral ammonium salt and capability of realizing complexation and release of chiral ammonium salt through an azo cis-trans isomerization behavior.
Owner:黄山市开发投资集团有限公司
Method for preparing super-pure nitric acid
InactiveCN101870460AEfficient removalSolve the problem of high content of impurity ionsNitric acidFiltrationDust particles
The invention discloses a method for preparing super-pure nitric acid. The method comprises the following steps: taking industrial nitric acid as a raw material; performing first stage filtration by a membrane filter consisting of dibenzo-18-crown-6 and solid-phase carrier composite membrane; performing second stage serial continuous rectification on the filtrate; collecting heavy waste acid and light waste acid respectively; and performing second stage filtration with the membrane filter to obtain the super-pure nitric acid serving as the target product. Upon analysis and detection, the content of each metal ion impurity is less than 1ppb, the dust particles bigger than 0.5microns are less than 5 / ml and the SEMI C8 standard is met. By performing the second stage filtration with the membrane filter consisting of the dibenzo-18-crown-6 and the solid-phase carrier composite membrane, the method overcomes the disadvantage of instable quality of the product in an existing method; the method effectively improves the purity of the super-pure nitric acid product by using the second stage serial continuous rectification technology; and the super-pure nitric acid product obtained by the method has stable quality and high purity and is suitable for scale continuous production.
Owner:SHANGAI HUAYI MICROELECTRONICS MATERIAL +1
Extractant composition including crown ether and calixarene extractants
An extractant composition comprising a mixed extractant solvent consisting of calix[4] arene-bis-(tert-octylbenzo)-crown-6 (“BOBCalixC6”), 4′,4′,(5′)-di-(t-butyldicyclo-hexano)-18-crown-6 (“DtBu18C6”), and at least one modifier dissolved in a diluent. The DtBu18C6 may be present at from approximately 0.01M to approximately 0.4M, such as at from approximately 0.086 M to approximately 0.108 M. The modifier may be 1-(2,2,3,3-tetrafluoropropoxy)-3-(4-sec-butylphenoxy)-2-propanol (“Cs-7SB”) and may be present at from approximately 0.01M to approximately 0.8M. In one embodiment, the mixed extractant solvent includes approximately 0.15M DtBu18C6, approximately 0.007M BOBCalixC6, and approximately 0.75M Cs-7SB modifier dissolved in an isoparaffinic hydrocarbon diluent. The extractant composition further comprises an aqueous phase. The mixed extractant solvent may be used to remove cesium and strontium from the aqueous phase.
Owner:BATTELLE ENERGY ALLIANCE LLC
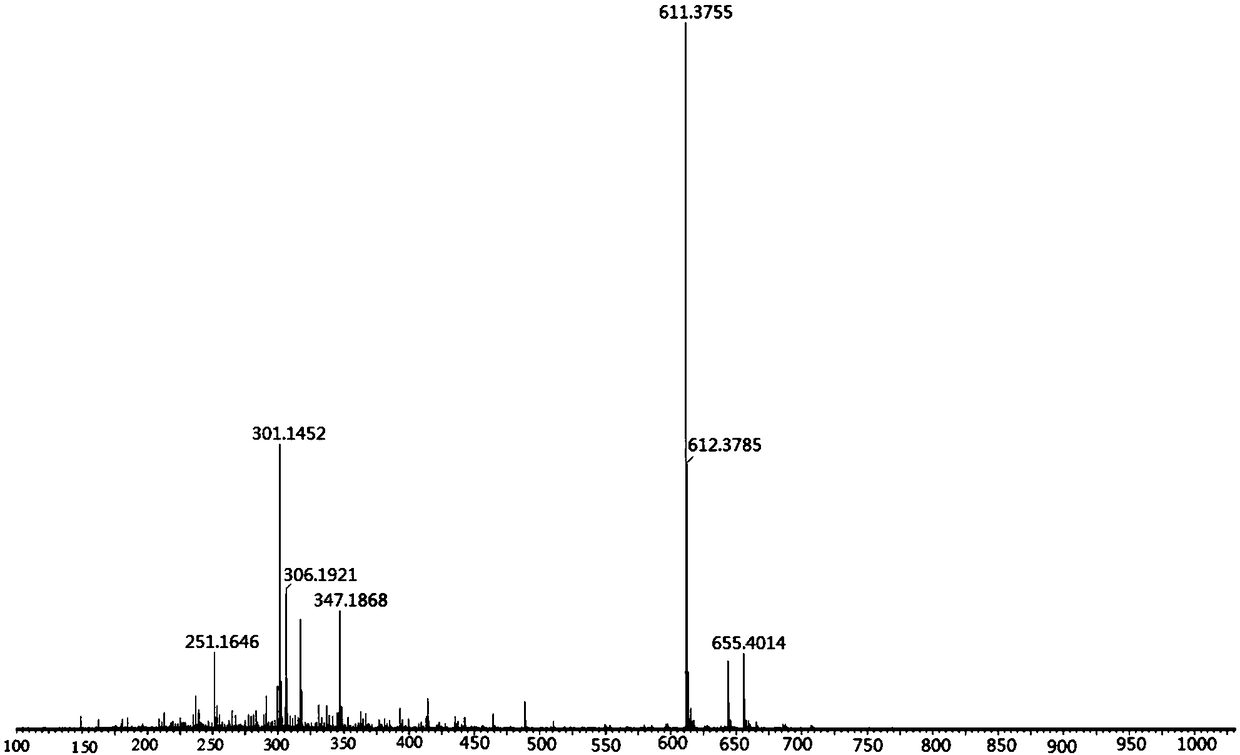
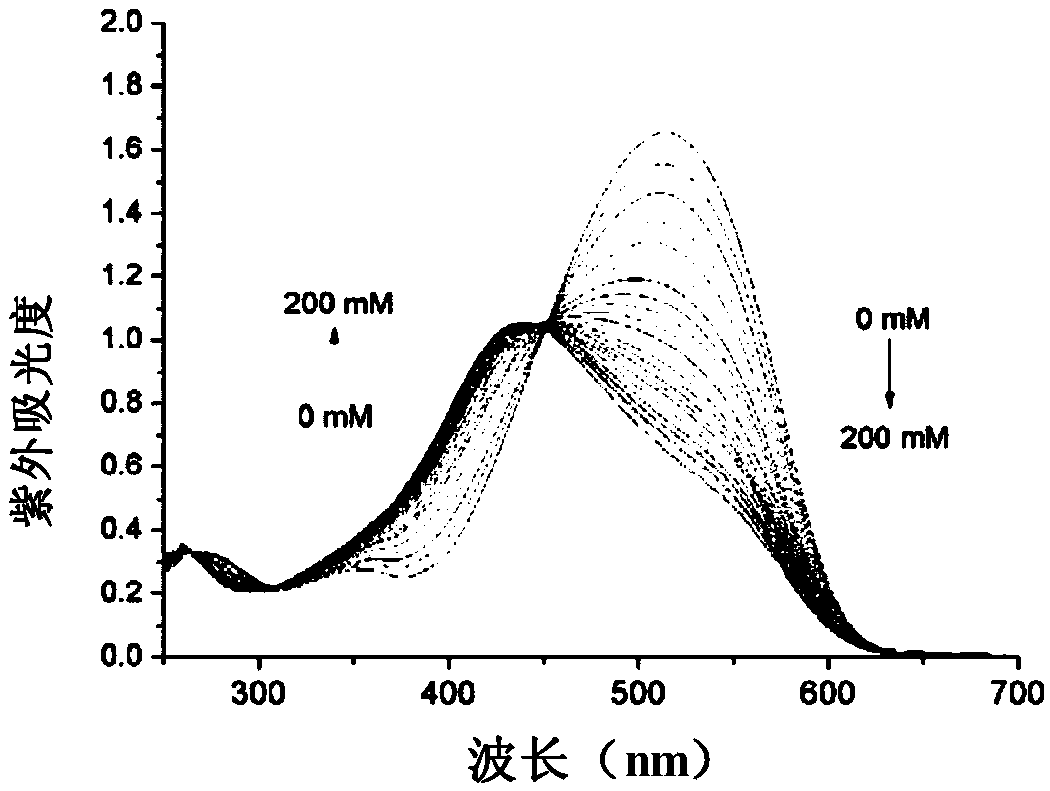
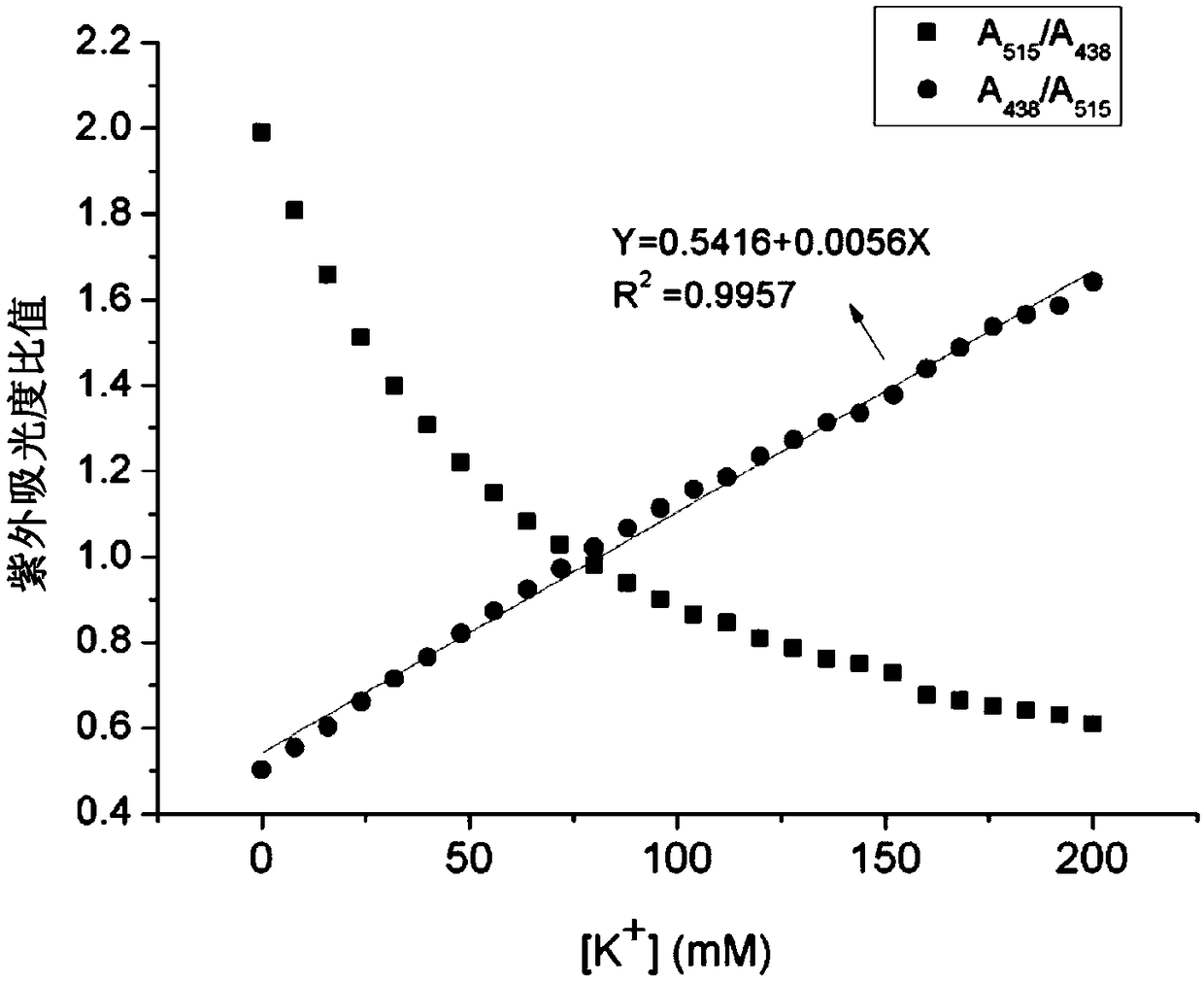
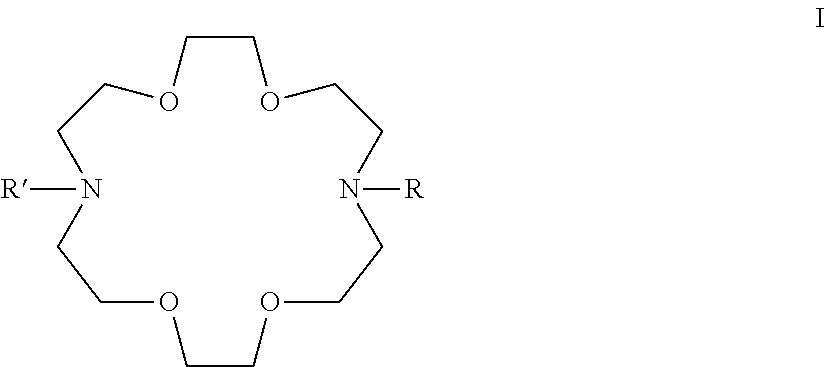
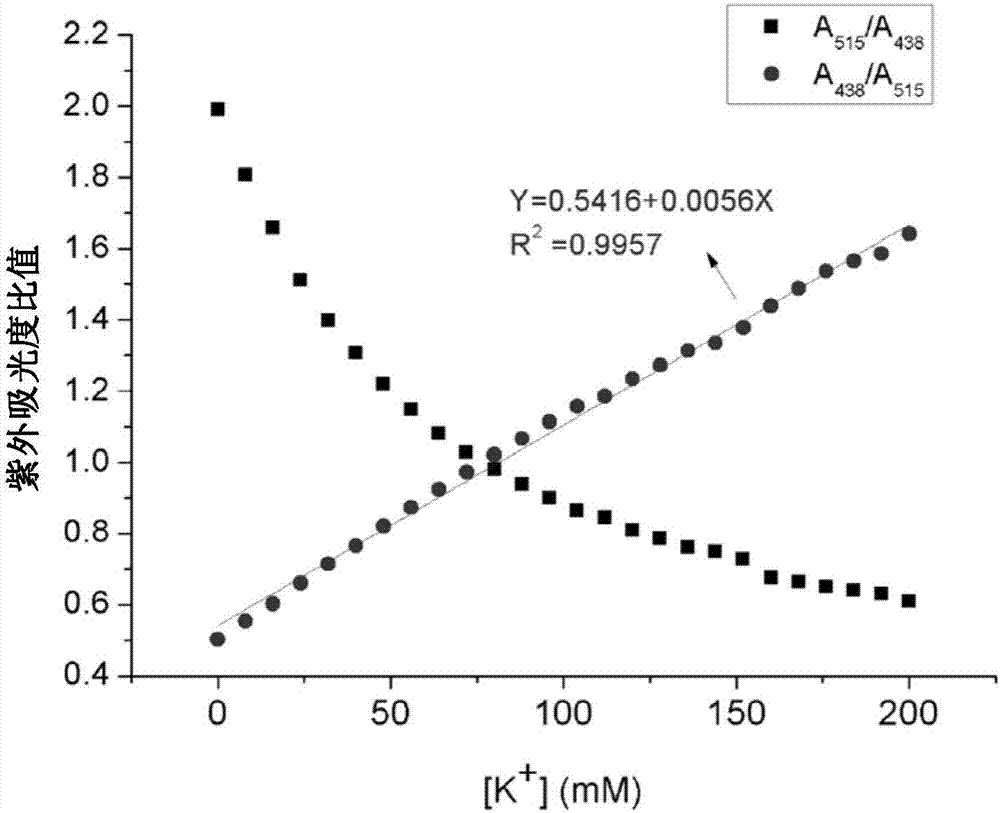
Potassium ion fluorescence probe as well as preparation method and application thereof
ActiveCN106929008AThe ability to donate electrons is weakenedGood ratio responseOrganic chemistryFluorescence/phosphorescenceSolubilityFluorescence
The invention provides a potassium ion fluorescence probe as well as a preparation method and application thereof. The potassium ion fluorescence probe utilizing phenylazepin-18-crown-6-amine as a recognition group and a hemicyanine dye group as a fluorescence group has the advantages of environmental sensitivity, good water solubility, high detection accuracy and high response speed to concentration change of potassium ions, is a colorimetric and instant rate type potassium ion detection probe and can be prepared into detection test paper, and the content of the potassium ions can be rapidly detected according to the color change of the test paper; and the potassium ion fluorescence probe is hopeful to be used for detecting the concentration of the potassium ions in traditional Chinese medicine injections and red wine, human urine or blood and has wide application prospects.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
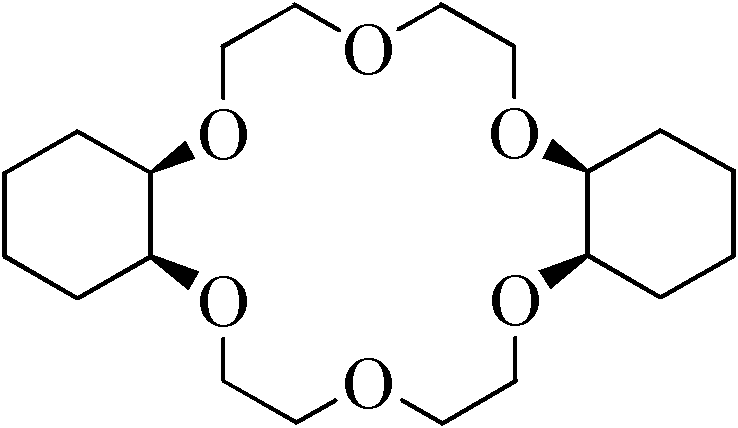
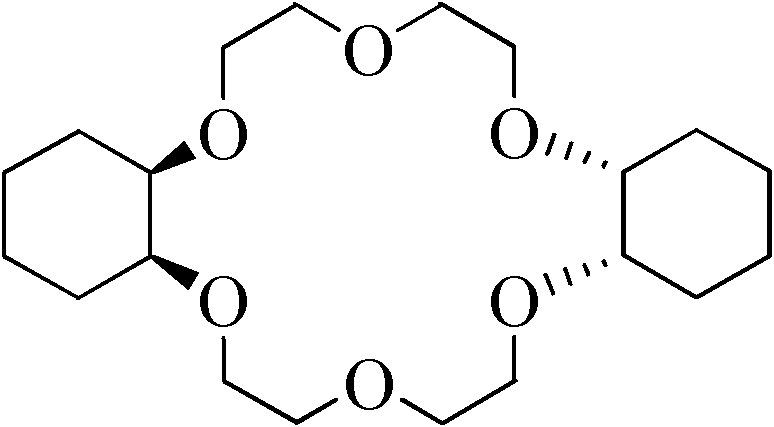
Synthesis method of dicyclohexyl-18-crown-6
InactiveCN102040584AEasy to prepareReduce difficultyOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsSynthesis methodsHydrogen pressure
The invention relates to a synthesis method of dicyclohexyl-18-crown-6 (DCH18C6), belonging to the technical field of preparation of an organic compound. The invention aims at increasing the content of cis-DCH18C6 isomer in the reaction product, thereby simplifying the purification process and improving the yield of cis-isomer. In the synthesis method, dibenzo-18-crown-6 is used as a raw material, and nano ruthenium metal powder is used as a catalyst to perform a catalytic hydrogenation reaction, wherein the reaction temperature is 100-200 DEG C, and the hydrogen pressure is 2-10 MPa. Although the method does not include any purification step, the content of the cis-isomer in the product can be up to 80% or above. The synthesis method has the characteristics of low cost and strong practicability and can be operated simply and conveniently.
Owner:TSINGHUA UNIV
Method for preparing 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol by one-pot process
InactiveCN102633661AImprove final yieldThe reaction steps are simpleOrganic compound preparationAmino-hyroxy compound preparationNitrogenEvaporation
The invention relates to a preparation method of cardanol oxyisopropanol, particularly a method for preparing 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol by a one-pot process. The invention aims to solve the problems of low yield, complex operation and high emulsification tendency in the existing method for preparing 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol. The method comprises the following steps: 1. preparing sodium cardanol; 2. adding [2,3]-(3-hydroxy-2-nitrogen)hydrindone-18-crown-6 and epoxy chloropropane to obtain a pre-reaction product; 3. adding diethanolamine to obtain a final reaction product; and 4. purifying sequentially by a rotary evaporation method, water-ethanol mixed solvent washing and silicagel column chromatography to obtain the fine 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol product. The invention is mainly used for preparing the 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol.
Owner:HARBIN NORMAL UNIVERSITY
Solvents for use in fluorination reactions
InactiveUS6198011B1Broaden applicationLow solvent contentPreparation by halogen replacementOrganic halogenationSolventOrganic compound
A method of fluorinating an organic compound comprising reacting an organic compound with a fluorinating agent characterized in that a perfluorocarbon compound is present in the reaction medium. The perfluorocarbon compound may replace an amount of a solvent which would otherwise be required for the reaction to proceed efficiently. The perfluorocarbon compound is readily recoverable after reaction and may be re-used in subsequent reactions. Additives to the reaction medium, such as 18-crown-6, may increase the amoun of solvent which may be replaced. The method is beneficial where solvent consumption would otherwise be large, or where solvent recovery would otherwise be difficult.
Owner:F2 CHEM LTD SPRINGFIELD WORKS
Amphiphilic pillar [5] arene self-assembled vesicle and depolymerization reversibility and controllability control method thereof
InactiveCN103980122AAchieve self-assemblyImprove mechanical propertiesOrganic compound preparationCarboxylic acid esters preparationDepolymerizationSynthesis methods
The invention discloses a synthesis method of low-polyethylene-glycol functional amphiphilic pillar [5] arene compound (AP5-glycol), wherein AP5-glycol is self-assembled in water to form a vesicle, and the vesicle can generate responsive depolymerization (the vesicle becomes small or gradually disappears) after being affected by external physical stimulation such as heating, ultrasonic treatment, violent stirring and the like and can be rapidly formed again after the external stimulation is stopped, i.e., the two processes including vesicle formation and vesicle depolymerization are reversible and controllable. Therefore, the vesicle has excellent thermodynamic reversibility and controllability. In addition, KPF6 and benzo-18-crown-6 can be respectively used as a switch for depolymerizing and forming a vesicle self-assembly again. The characteristics can ensure that the vesicle has outstanding recyclability in application such as drug release or transfer and the like.
Owner:NANTONG VOCATIONAL COLLEGE
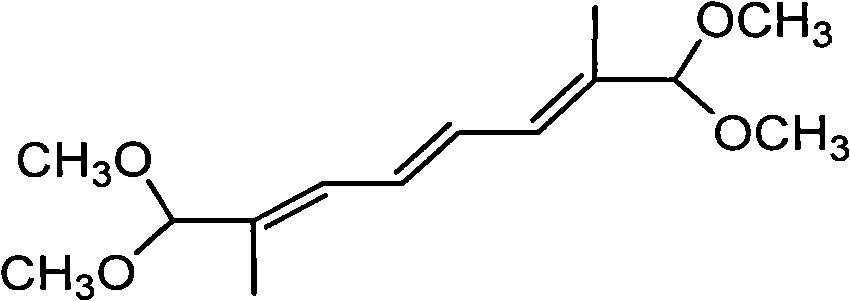
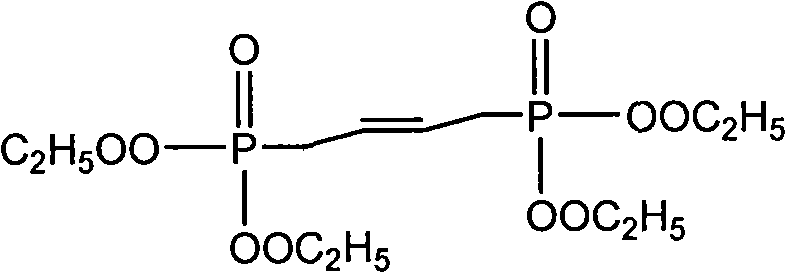
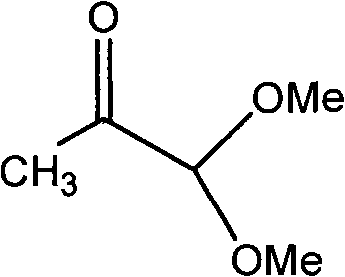
New synthesis process of 1,1,8,8-tetramethoxy-2,7-dimethyl-2,4,6-octatriene
InactiveCN101597220ARealize industrializationHigh yieldOrganic chemistryOrganic compound preparationReaction system18-Crown-6
The invention discloses a new synthesis process of 1,1,8,8-tetramethoxy-2,7-dimethyl-2,4,6-octatriene. The original process is improved; wherein, alkali metal hydroxide or alkali metal alkylate is fixed on the carrier which is added into the reaction system and crown ether of 18-crown-6 or 12-crown-4 is added as catalyst, thus obviously improving the yield of 1,1,8,8-tetramethoxy-2,7-dimethyl-2,4,6-octatriene.
Owner:GUANGZHOU WISDOM BIO TECH
Production process for ultra-pure N-methylpyrrolidone
InactiveCN102399179AEfficient removalSimple and fast operationOrganic chemistryFiltrationTechnical grade
The invention relates to a production process for ultra-pure N-methylpyrrolidone. According to the invention, industrial grade N-methylpyrrolidone is used as a raw material and is subjected to pretreatment, 4A molecular sieve adsorption and dehydration and two times of membrane filtration respectively through a beta-cyclodextrin composite membrane and a 18-crown-6 composite membrane; an obtained filtrate undergoes vacuum rectification; collected fractions are subjected to condensation at first and to third-stage membrane filtration so as to prepare a target product. Ultra-pure N-methylpyrrolidone prepared by the process provided in the invention has a purity of more than 99.8%, water content of less than 0.03% and individual metal ion content of less than 1 ppb, being in conformity with the chemical material part class 8 (SEMI C8) established by Semiconductor Equipment and Materials International. Compared to the prior art, the invention has the advantages of stable product quality, simple operation and suitability for industrial continuous production.
Owner:SHANGHAI CHEM REAGENT RES INST
Alkaline agent for dye printing and its production process
InactiveCN1970880AEasy to wash offReduce labor intensityDyeing processCarboxylic acidPotassium carbonate
The invention relates to a caustic alkali for printing and dyeing, characterized in that it comprises 10%-20% by wt of disodium hydrogen phosphate, 15%-30% by wt of sodium hydroxide, 5%-18% by wt of sodium carbonate, 1%-10% by wt of potassium carbonate, 0.1%-2% by wt of organic ene acid, 1.6%-16% by wt of organic phosphonic acid, 0.01%-5% by wt of organic carboxylic acid, 0.01%-2% by wt of crown ether and water. The manufacturing method for said caustic alkali for printing and dyeing contains that organic phosphonic acid, organic carboxylic acid, organic ene acid and water are poured into the reaction vessel and mixed evenly; the temperature is increased to 120-140DEG C gradually; K+18-crown-6 crown ether is dropped into it, the mixture is mixed round and the temperature is decreased to normal temperature; then sodium hydroxide is added into it and the temperature is increased to 120-140DEG C; after that sodium carbonate is added into it and the temperature is maintained to air discharging; finally disodium hydrogen phosphate is added into and the temperature is maintained for 6-8 hours.
Owner:上海瑞鹰化工有限公司 +1
Method for producing of ultra-clean and high-purity n-methyl pyrrolidone
InactiveUS20120071670A1Efficient removalQuality productionOrganic chemistryFiltrationBeta-Cyclodextrins
The present invention provides a method for producing of ultra-clean and high-purity N-methyl pyrrolidone through using industrial grade N-methyl pyrrolidone as raw material. After the pretreatment, sorption and dehydration with 4A molecular sieve, twice membrane filtrations are carried out through using β-cyclodextrin composite membrane for the first and 18-crown-6 composite membrane for the second. The filtrate is rectified under vacuum and filtered through using complexant composite microporous membrane to obtain the product. The ultra-clean and high-purity N-methyl pyrrolidone, produced by the method provided by the present invention, is up to the SEMI C8 standard. And the purity of the product is over 99.8%, the moisture content is less than 0.03%, and the content of single metal ion is less than 1 ppb. Comparing to the prior art, the present invention has the advantage such as the stable quality of the product, simple operation, and is suitable for industrial continuous production.
Owner:SHANGHAI CHEM REAGENT RES INST
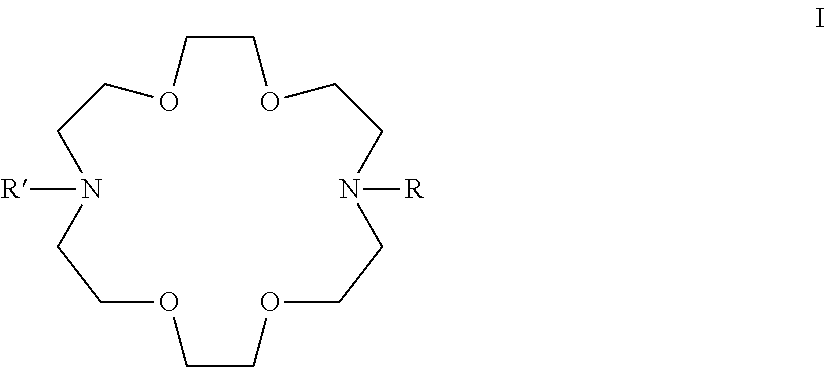
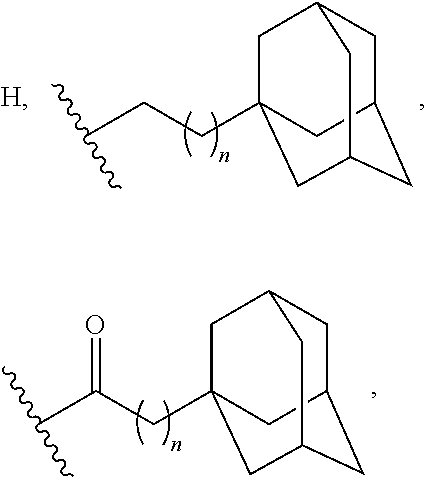
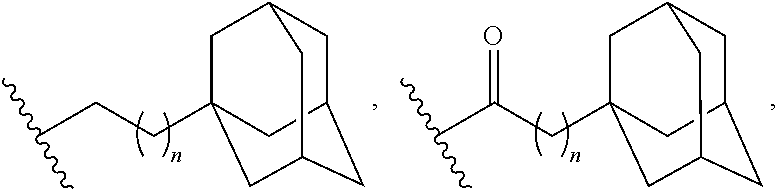
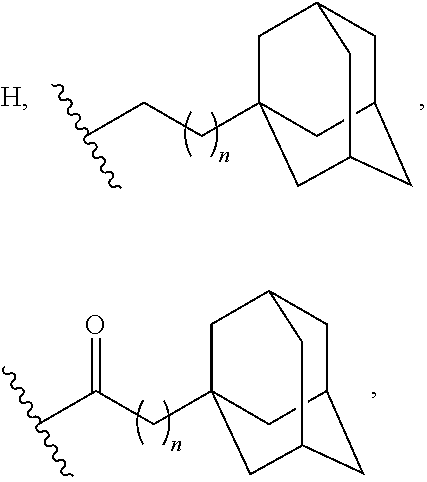
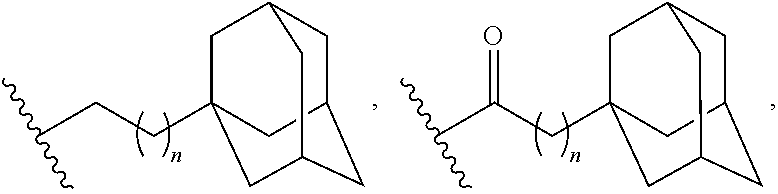
Adamantane Derivatives Of Aza-Crown Ethers And Their Use In Treatment Of Tumor
The invention relates to adamantane diaza-crown ether derivatives and the use of mono and diaza-crown ether adamantine derivatives in treatment, especially in tumor treatment. Adamantane aza-crown ethers were obtained by reaction of the corresponding adamantane derived tosylates or adamantane acid chlorides with mono- and diaza-18-crown-6. The prepared compounds showed moderate (monoaza-18-crown-6) to strong (diaza-18-crown-6) antiproliferative and cytotoxic activity on several tumor cell lines, revealing their potential for inhibiting the growth of other tumor cells.
Owner:RUDJER BOSKOVIC INST
Preparation method of organic/layered double hydroxide (LDH) complex
InactiveCN102225917AHigh crystallinityHigh affinityMaterial nanotechnologyOrganic chemistryCrystallinitySolvent
The invention provides a preparation method of an organic / layered double hydroxide (LDH) complex. The preparation method comprises the following steps of a) dissolving 1,10-dioxo-4,7,13, 16-tetranitro-18-crown-6 in a CH2Cl2 solvent, adding anhydrous potassium carbonate into the solution and mixing them, heating and refluxing the mixed solution, then adding ethyl bromoacetate into the mixed solution and refluxing it, then filtering and evaporating the refluxed solution to obtain an oil-like substance, adding hydrochloric acid solution into the oil-like substance, heating, refluxing and filtering the mixed solution, and evaporating the filtrate to make crystals be separated out to obtain 4,7,13,16-tetracarboxymethyl-1,10-dioxo-4,7,13, 16-tetraazaoctadecane (TECA), and b) mixing TECA, NaOH and methanamide, adding MgAl-NO3-LDH into the mixture, standing for 10 minutes to 16 hours, and then centrifuging, washing and drying the mixture to obtain a TECA / LDH complex, wherein a mass ratio of TECA to MgAl-NO3-LDH is 1. Through the preparation method, a TECA / LDH complex can be prepared in a short reaction time thus a production period of the TECA / LDH complex is saved; corrosive effects of methanamide on LDH laminates are reduced and compositions of the laminates are maintained well; and a crystallinity and a yield of complex products are improved.
Owner:BEIJING NORMAL UNIVERSITY +1
Preparation of 2,2,2- trifluoroethyl-1,1,2,3,3,3-hexafluoroisopropyl ether
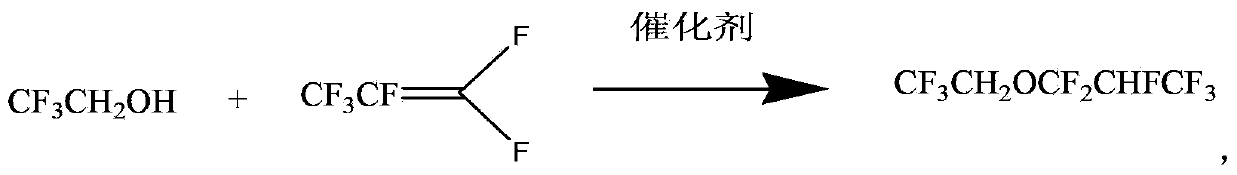
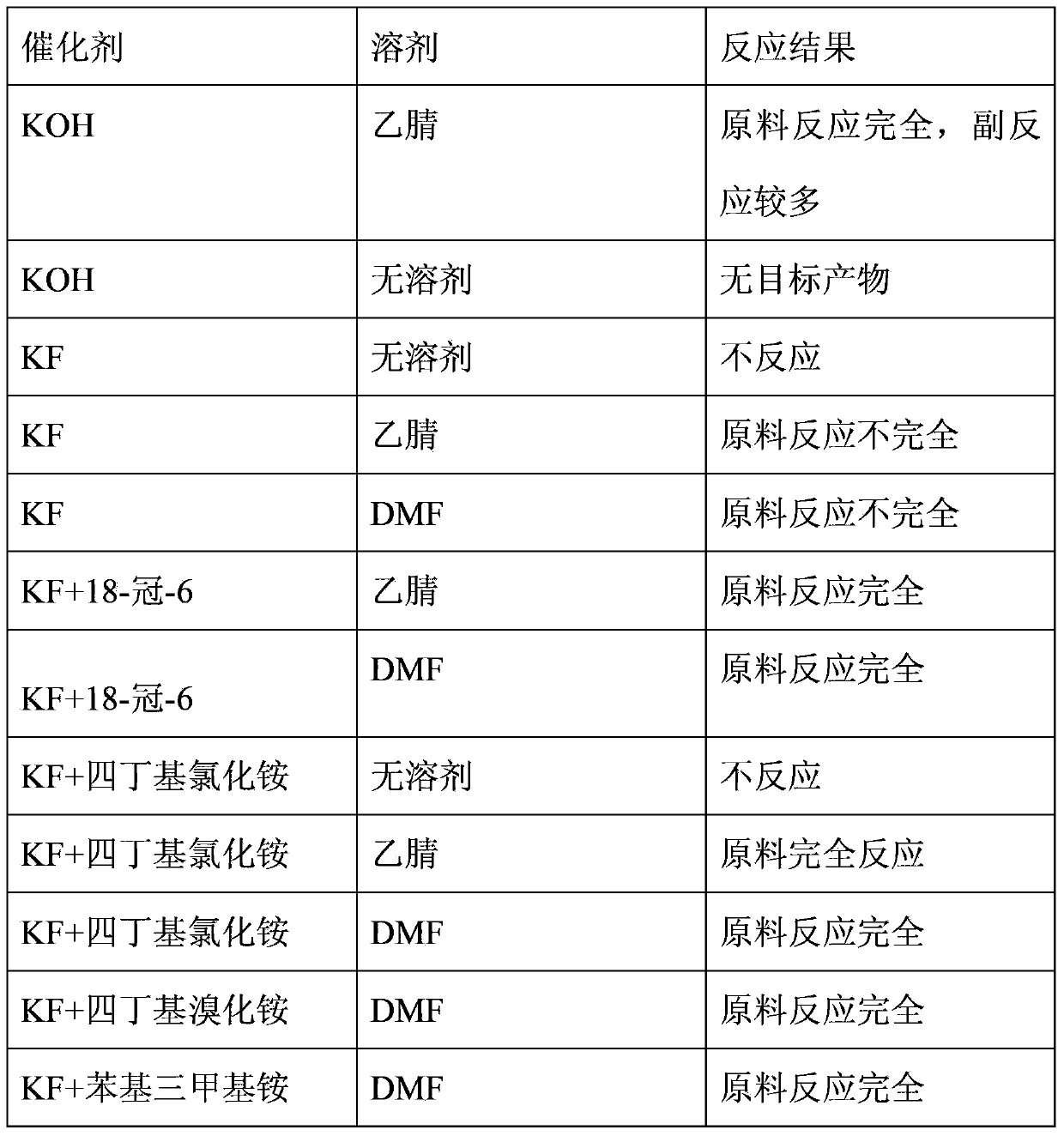
InactiveCN103360222ALow priceMild conditionsEther preparation by compound additionPotassium fluorideHexafluoropropylene
The invention relates to a preparation method of 2,2,2-trifluoroethyl-1,1,2,3,3,3-hexafluoroisopropyl ether. Trifluoroethanol reacts with hexafluoropropylene in an organic polar solvent and in the presence of potassium fluoride and phase-transfer catalyst, at the temperature of room temperature to 50 DEG C, so as to produce 2,2,2- trifluoroethyl-1,1,2,3,3,3-hexafluoroisopropyl ether, and the phase-transfer catalyst is 18-crown-6, tetrabutylammonium chloride or trimethylphenylammonium. The preparation method of 2,2,2- trifluoroethyl-1,1,2,3,3,3-hexafluoroisopropyl ether has the advantages of mild reaction conditions, normal pressure reaction, low catalyst price, convenient post-treatment, and good yield and selectivity, and is suitable for industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
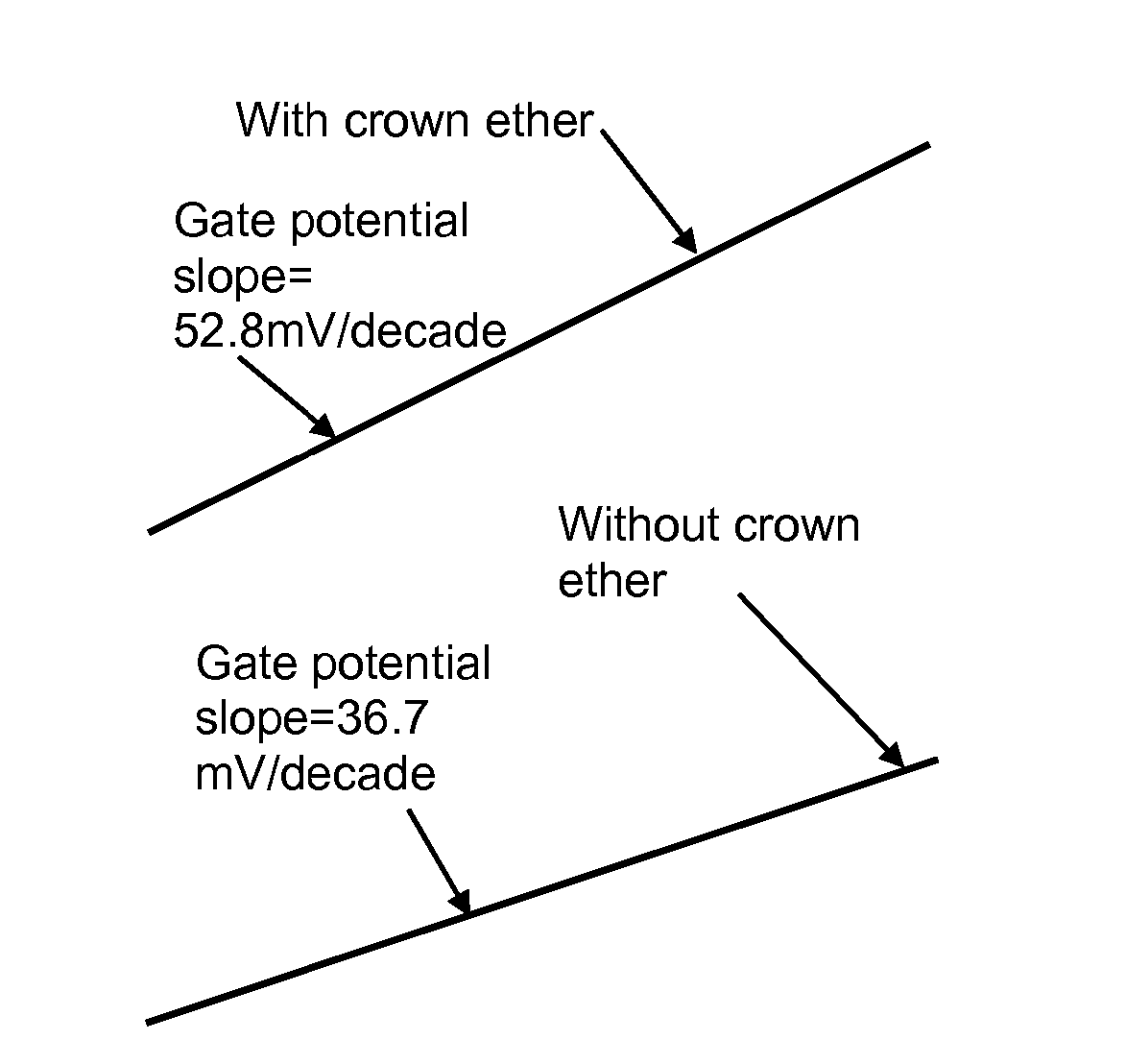
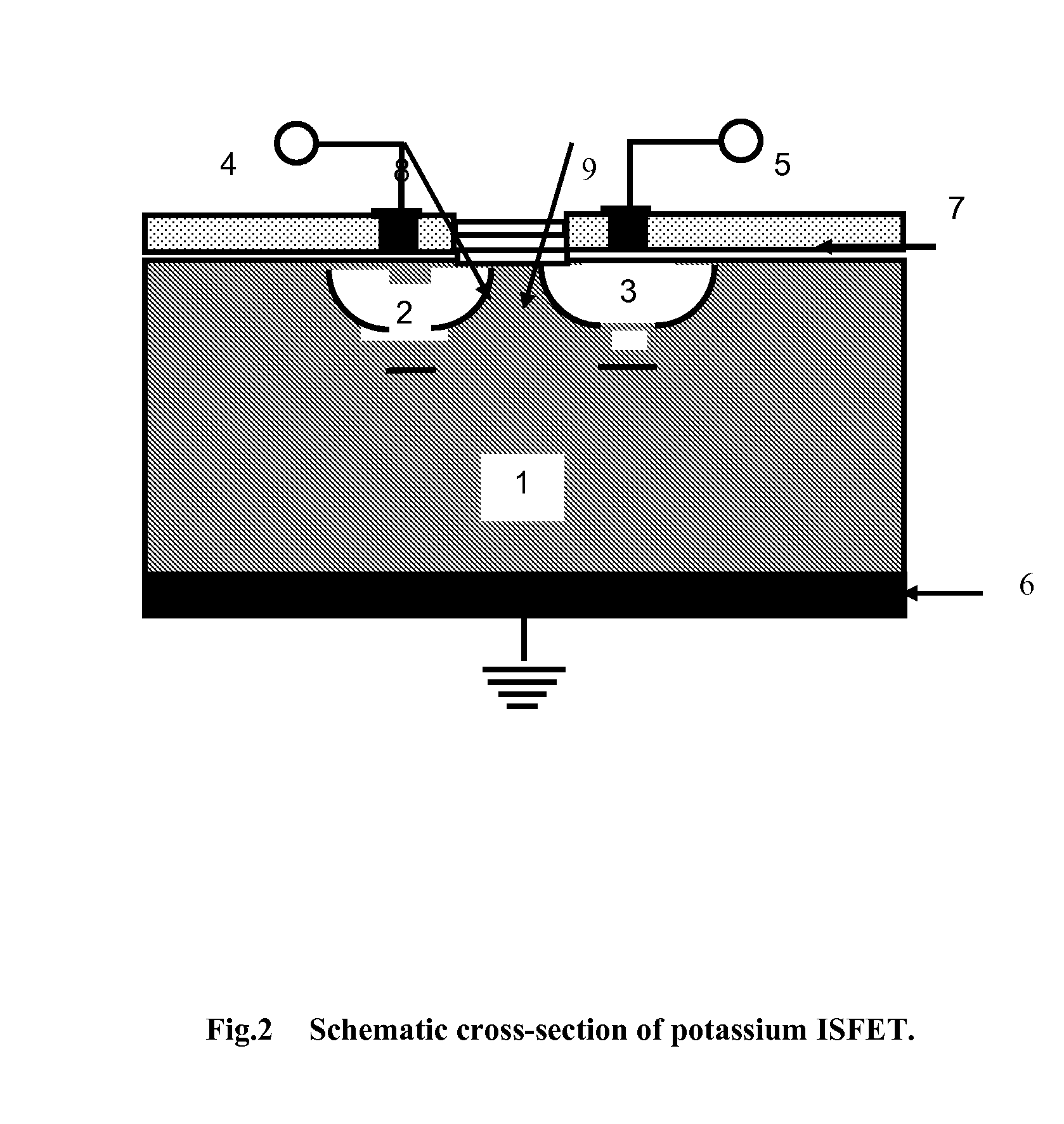
Biosensor to determine potassium concentration in human blood serum
InactiveUS20070227886A1Select potassiumAccurate inductionImmobilised enzymesBioreactor/fermenter combinationsHigh concentrationIon selective field effect transistor
The present invention relates to the development of a biosensor to determine potassium in human blood serum using dibenzo-18-crown-6 (DB18C6) as ionophore. Human blood serum contains potassium in ppm levels i.e. 137 to 200 mg / litre and sodium co exists with a 30 times higher concentration. Such a high concentration tends to interfere the selectivity towards potassium, but DB18C6 proves to have an excellent selectivity towards potassium and is highly sensitive to the lowest concentration of potassium levels present in the human blood serum. So the present invention reports the fabrication and characterization of ISFET (Ion Selective Field Effect transistor) coated with a monolayer of crown ether, dissolved in chloroform, on the gate of electrode.
Owner:COUNCIL OF SCI & IND RES
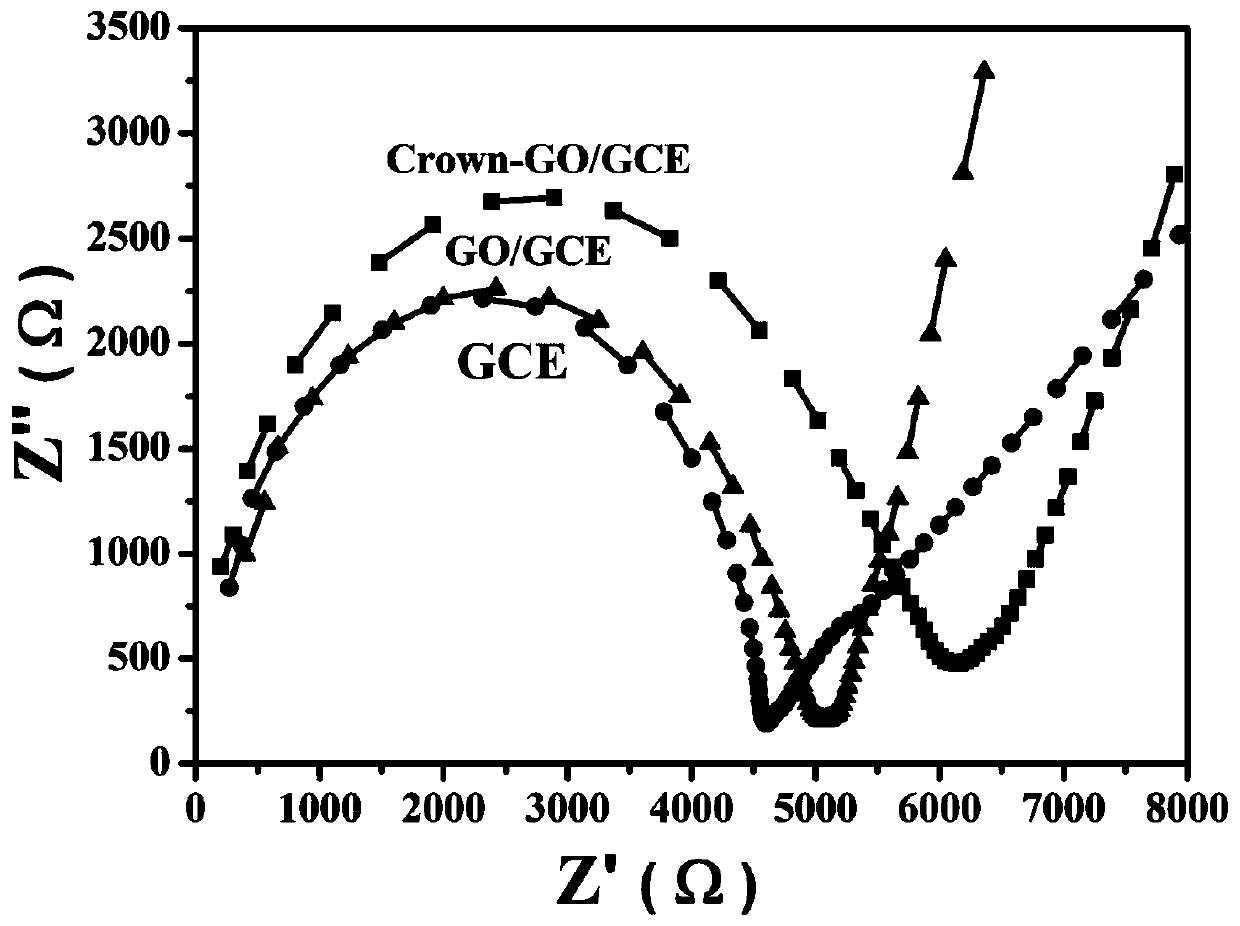
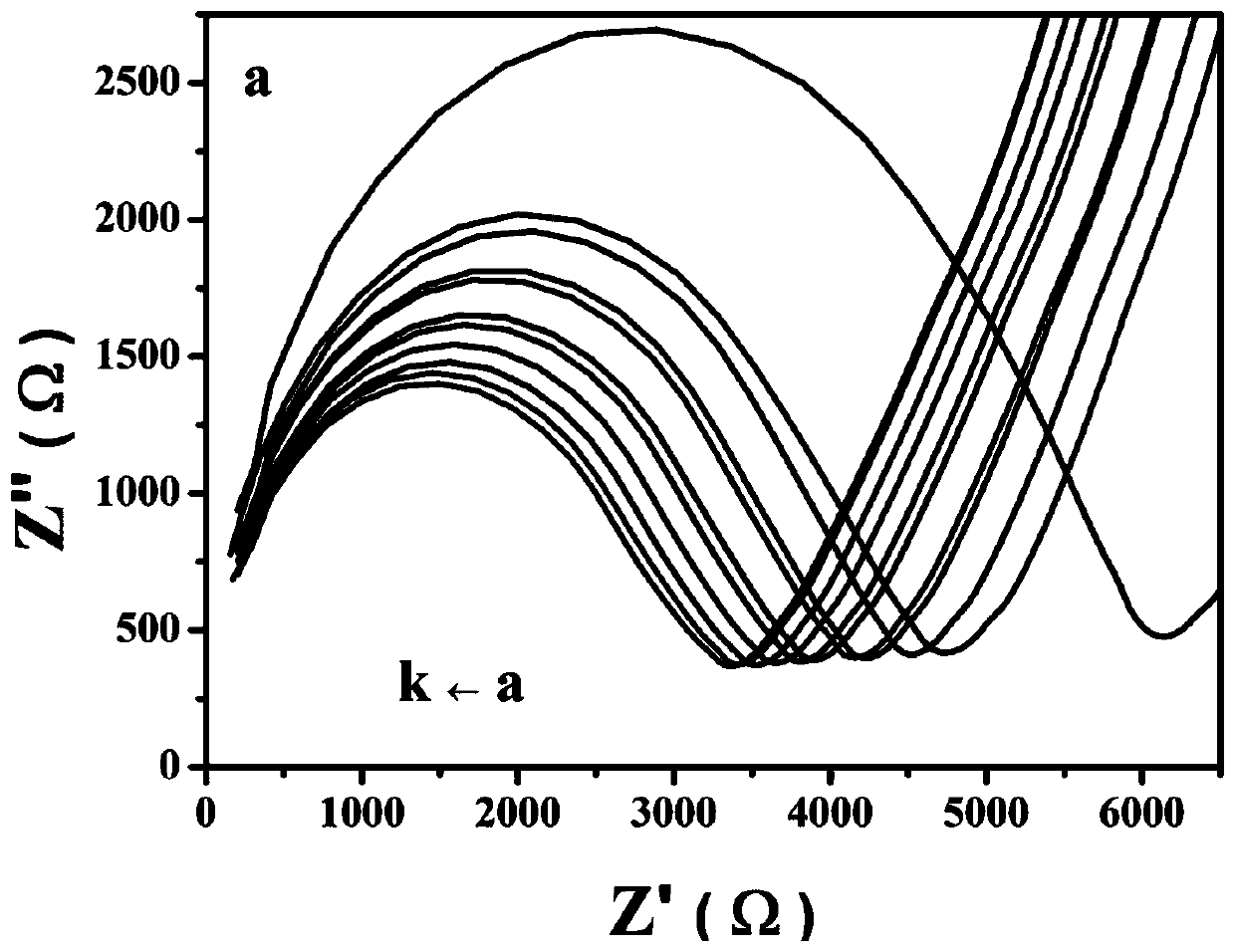
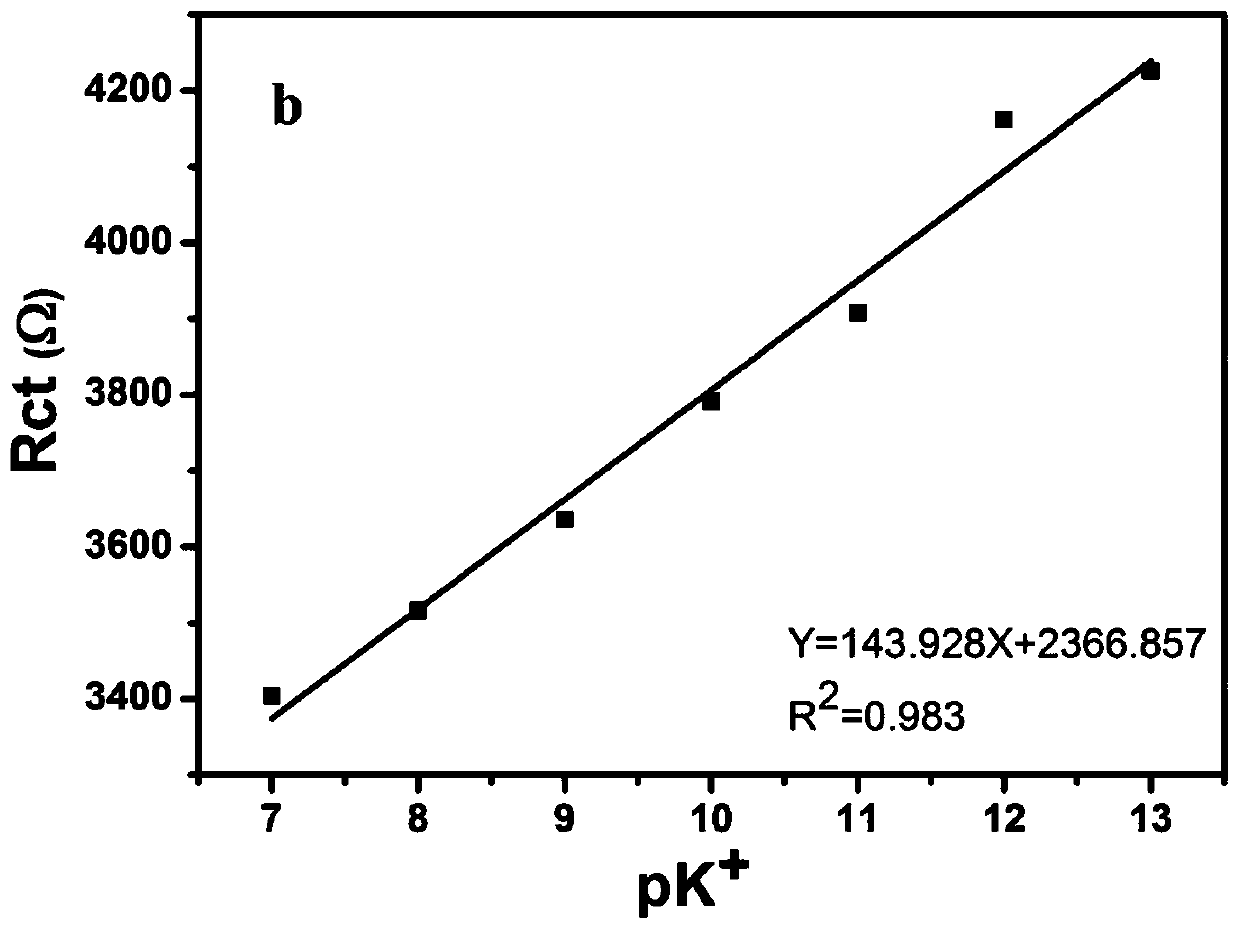
Electrochemical sensor and potassium ion detection method thereof
InactiveCN110146577AInhibition of dissolutionInhibitionMaterial analysis by electric/magnetic meansElectricityPotassium ions
The invention discloses an electrochemical sensor and a potassium ion detection method thereof, and belongs to the field of analysis and detection. A preparation method for a work electrode of the electrochemical sensor comprises that 1-aza-18-crown-6 functionalized oxidized graphene (Crown-GO) is dispersed in an aqueous solution by supersonics to obtain a Crown-GO suspension; and the Crown-GO suspension is uniformly dropped on the surface of a glassy carbon electrode after polishing, cleaning and blowing-dry to obtain Crown-GO / GCE. It is shown, by electrochemical performance test, the prepared glassy carbon electrode modified by the Crown-GO composite material can be used to detect potassium ions highly sensitively with low detection limit and wide detection range; and the prepared electrochemical sensor has the advantages of being simple in the electrode material preparation process, friendly to the environment, simple in operation and high in sensitivity, and can be widely applied in the potassium ion detection field.
Owner:TAIYUAN UNIV OF TECH
Continuous producing technique for ultra-high pure nitric acid
The invention relates to a process for continuously producing extra high purity nitric acid, which comprises the following steps: first, industrial grade 80-90% nitric acid raw material and double allyl 18-crown-6 ether organosilicon macromolecular complexing agent accounting for 0.2-2% of the weight of nitric acid raw material are mixed in a pretreater, then, the mixture is filtered by a micro-filtration membrane under a working pressure of 0.1-0.2 Mpa, the filtrate enters a rectification tower, and the semi-finished product going through the rectification tower is diluted by ultra pure waterin a dilution device; after the dilution is finished, the dissociative NO2 is expelled by high pure nitrogen in a whitening device, and the obtained finished product is filtered by a nanometer filtermembrane and enters a finished product receiver under the working pressure of 0.5-0.8 Mpa; wherein, the aperture of the micro filtration membrane is 0.2 to 0.8 Mu m, and the aperture of the nanometerfilter membrane is 0.5 to 1.5 nm. In the prepared ultra high purity nitric acid, the content of single cation is lower than 1 ppb, the content of single anion is lower than 100 ppb, and the content of the dust particle larger than 0.5 Mu m is lower than 5 per milliliter. The production process for extra high purity nitric acid has the advantages of simple technique, low production costs, high purityof products, low content of impurity ions, and applicability for large-scale mass production.
Owner:JIANGYIN RUNMA ELECTRONICS MATERIAL
A kind of potassium ion fluorescent probe and its preparation method and application
ActiveCN106929008BGood water solubilityImprove detection accuracyOrganic chemistryFluorescence/phosphorescenceSolubilityFluorescence
The invention provides a potassium ion fluorescence probe as well as a preparation method and application thereof. The potassium ion fluorescence probe utilizing phenylazepin-18-crown-6-amine as a recognition group and a hemicyanine dye group as a fluorescence group has the advantages of environmental sensitivity, good water solubility, high detection accuracy and high response speed to concentration change of potassium ions, is a colorimetric and instant rate type potassium ion detection probe and can be prepared into detection test paper, and the content of the potassium ions can be rapidly detected according to the color change of the test paper; and the potassium ion fluorescence probe is hopeful to be used for detecting the concentration of the potassium ions in traditional Chinese medicine injections and red wine, human urine or blood and has wide application prospects.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Preparation method of polyether macromonomer for polycarboxylate water reducer
The invention discloses a preparation method of a polyether macromonomer for a polycarboxylate water reducer. The preparation method comprises the following steps: (1) taking a part of methyl allyl alcohol to react with a catalyst one, then putting a reacted product into the remaining methallyl alcohol, and introducing ethylene oxide and propylene oxide for reaction, so that a methylallyl random oligomer is obtained; and (2) allowing the methylallyl random oligomer to react with a catalyst two and a catalyst three, and then introducing ethylene oxide and propylene oxide for reaction; wherein the catalyst one is one or more of sodium, potassium and sodium hydride; the catalyst two is one or more of 18-crown-6 and 15-crown-5; and the catalyst three is phosphonitrilic chloride trimer. Compared with the prior art, the preparation method of the polyether macromonomer for the polycarboxylate water reducer has the advantages that the effective content is high, the by-product content is low, the molecular weight distribution is narrow, the double bond retention rate is high, and the likes, wherein a molecular weight distribution coefficient is less than 1.05, and the double bond retentionrate is greater than 98.0%.
Owner:ZHEJIANG HUANGMA TECH +3
Adamantane derivatives of AZA-crown ethers and their use in treatment of tumor
Owner:RUDJER BOSKOVIC INST
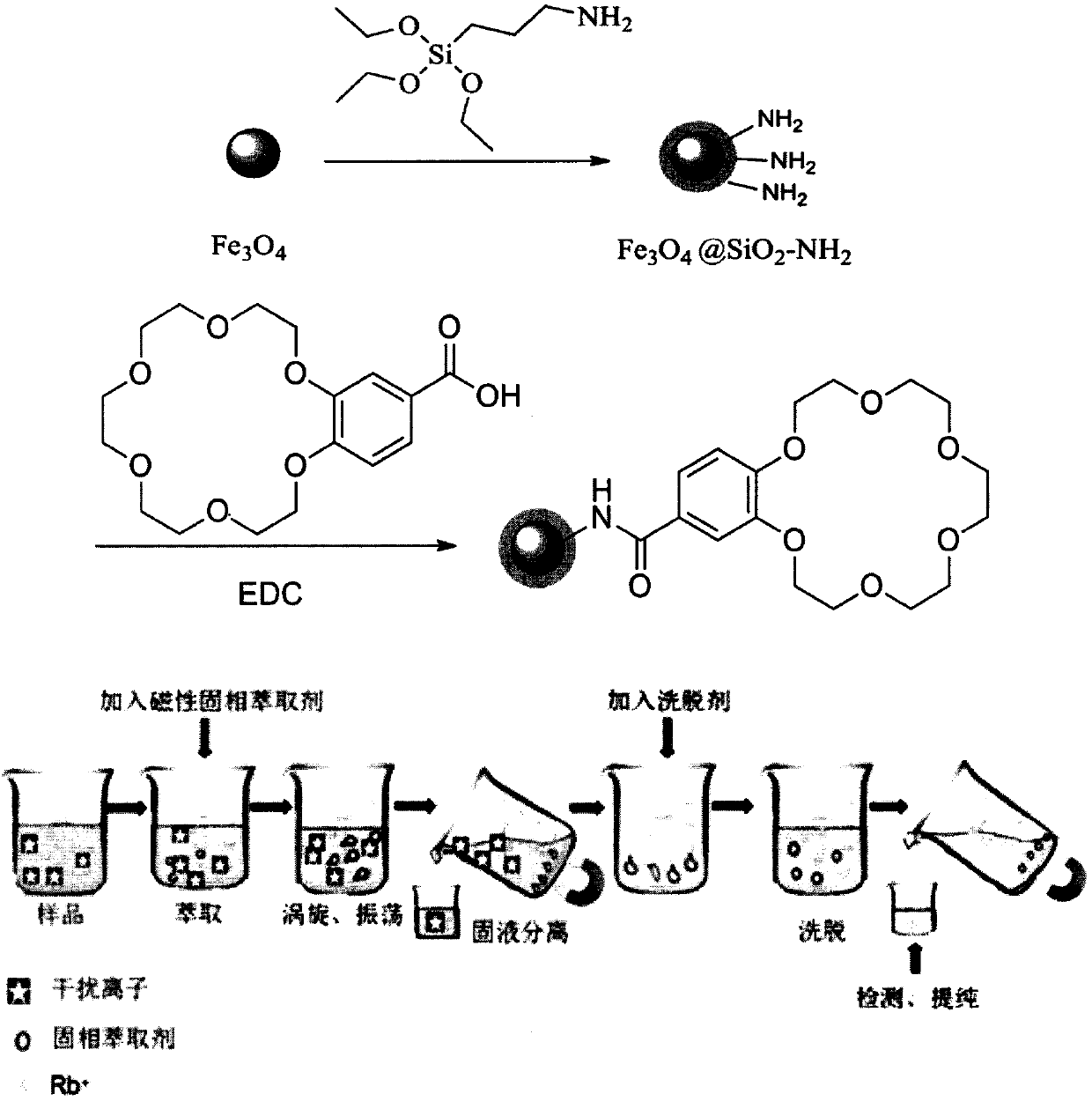
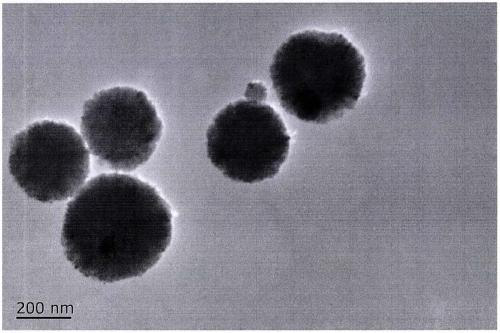
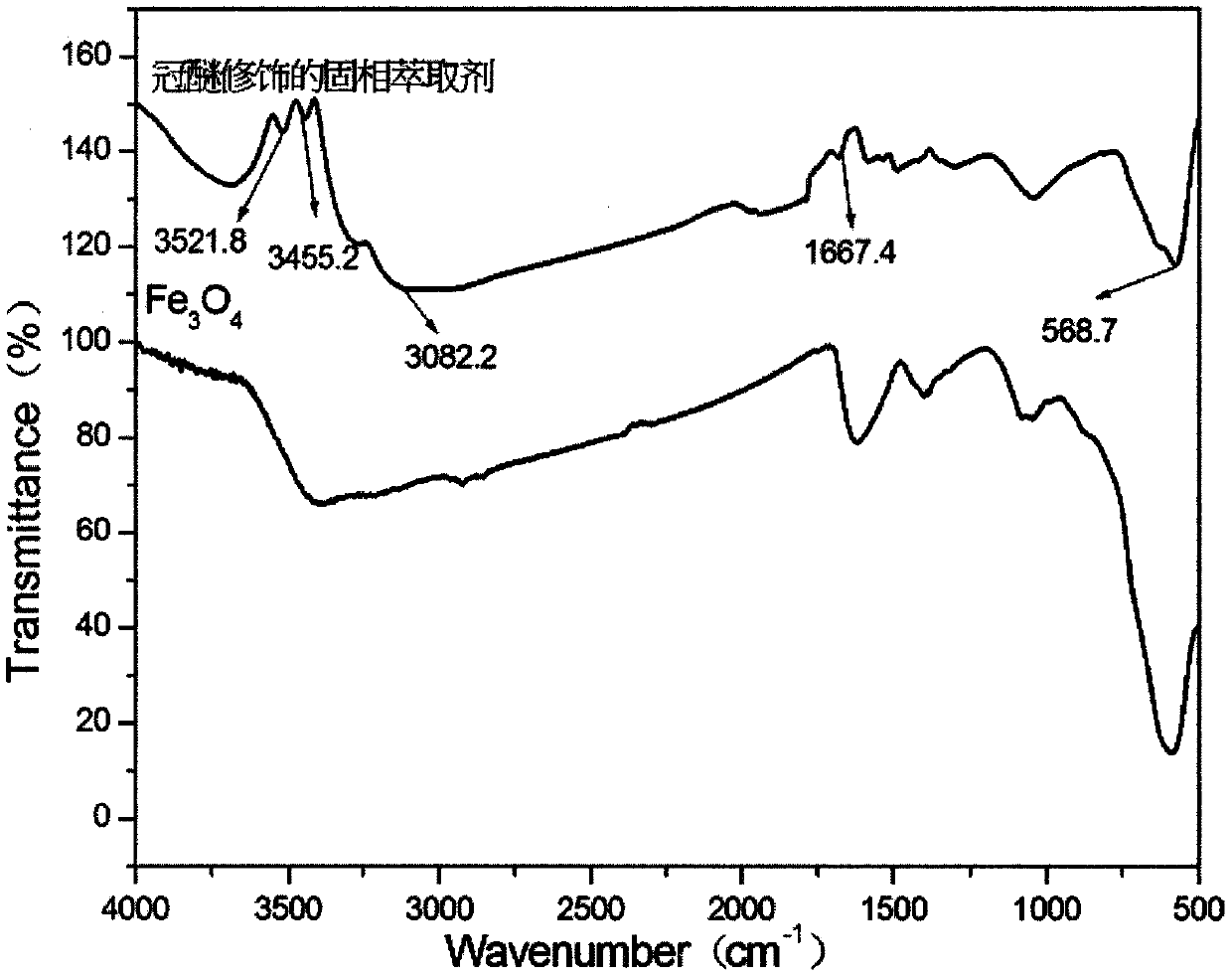
Preparation method of amino and 18-crown-6 modified magnetic solid-phase extraction agent based on rubidium extraction
InactiveCN109865312AEasy to separateImprove hydrophilicityOther chemical processesLiquid solutions solvent extractionRubidiumSolid phase extraction
The invention discloses a preparation method of an amino and 18-crown-6 modified magnetic solid-phase extraction agent based on rubidium extraction. According to the preparation method, ferroferric oxide is taken as a magnetic core, and aminopropyl triethoxysilane and 4-carboxybenzo-18-crown-6 are taken as reaction raw materials. According to the preparation method disclosed by the invention, selectivity of rubidium hydrate ions is enhanced by using a synergistic effect of 18-crown-6 and amino; the magnetic property of ferroferric oxide is utilized, so that the purpose of conveniently and rapidly separating rubidium ions from a complex matrix containing a large amount of alkali metal ions is achieved under an additional magnetic field; a prepared solid-phase extraction agent is used for separating and enriching rubidium in a water solution, so that pretreatment time of a sample is shortened; the enrichment factor of the rubidium ions can reach 100 times or above, selectivity of the extraction agent to rubidium is good, and interfering ions such as K<+>, Na<+> and the like can almost be completely removed, so that background interference is eliminated, the content of rubidium can bedetected only by a conventional atomic absorption method, and detection precision is greatly improved.
Owner:QINGHAI UNIVERSITY
Extractant compositions for co-extracting cesium and strontium, a method of separating cesium and strontium from an aqueous feed, and calixarene compounds
Owner:BATTELLE ENERGY ALLIANCE LLC +1
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide](https://images-eureka.patsnap.com/patent_img/513cbca7-c8d0-42d7-a898-6e6990555546/DSA00000351862100011.png)
![Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide](https://images-eureka.patsnap.com/patent_img/513cbca7-c8d0-42d7-a898-6e6990555546/FSA00000351862200011.png)
![Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide](https://images-eureka.patsnap.com/patent_img/513cbca7-c8d0-42d7-a898-6e6990555546/FSA00000351862200021.png)
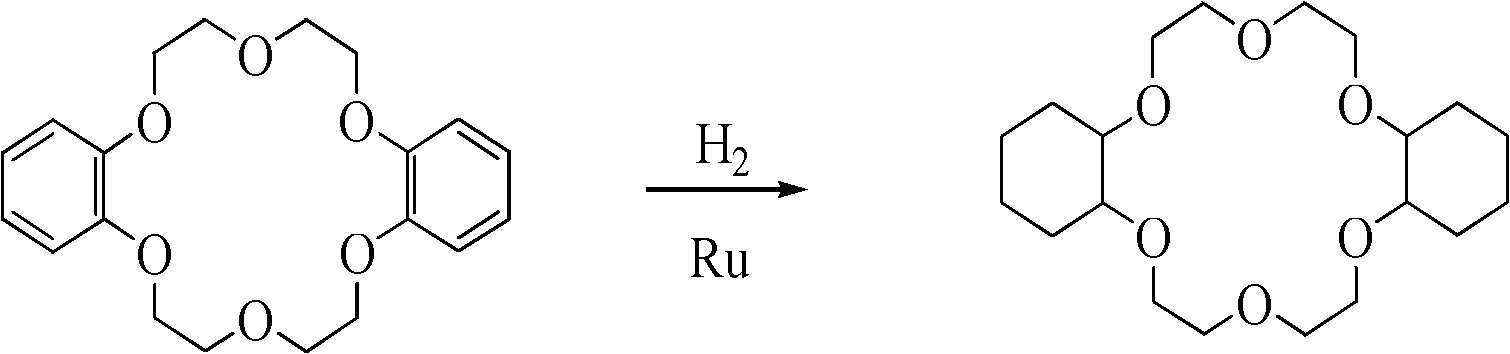
![Method for preparing 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol by one-pot process Method for preparing 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol by one-pot process](https://images-eureka.patsnap.com/patent_img/c16f69bb-062a-43a1-a1fb-7021dda72800/HDA0000147905420000011.PNG)
![Method for preparing 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol by one-pot process Method for preparing 1-[di-(2-hydroxyethyl)amino]-3-cardanol oxyisopropanol by one-pot process](https://images-eureka.patsnap.com/patent_img/c16f69bb-062a-43a1-a1fb-7021dda72800/EDA0000147905440000011.PNG)
![Amphiphilic pillar [5] arene self-assembled vesicle and depolymerization reversibility and controllability control method thereof Amphiphilic pillar [5] arene self-assembled vesicle and depolymerization reversibility and controllability control method thereof](https://images-eureka.patsnap.com/patent_img/606d4862-338b-48d2-b936-089ed3044858/140523135951.PNG)
![Amphiphilic pillar [5] arene self-assembled vesicle and depolymerization reversibility and controllability control method thereof Amphiphilic pillar [5] arene self-assembled vesicle and depolymerization reversibility and controllability control method thereof](https://images-eureka.patsnap.com/patent_img/606d4862-338b-48d2-b936-089ed3044858/140523135957.PNG)
![Amphiphilic pillar [5] arene self-assembled vesicle and depolymerization reversibility and controllability control method thereof Amphiphilic pillar [5] arene self-assembled vesicle and depolymerization reversibility and controllability control method thereof](https://images-eureka.patsnap.com/patent_img/606d4862-338b-48d2-b936-089ed3044858/140523140003.PNG)