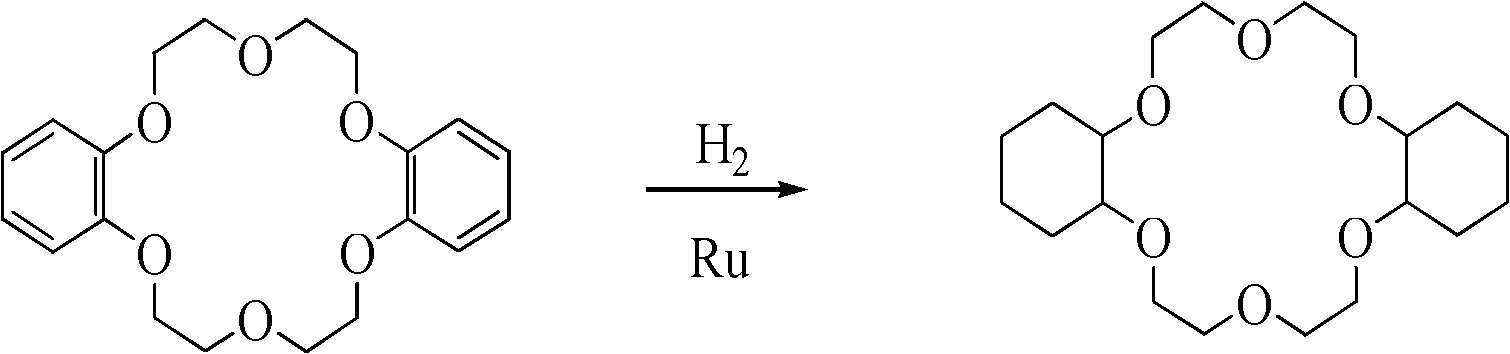Synthesis method of dicyclohexyl-18-crown-6
A technology of dicyclohexyl and synthesis method, which is applied in the field of organic compound preparation, can solve problems such as low content and large product loss, and achieve the effect of reducing difficulty and making the preparation method simple and feasible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
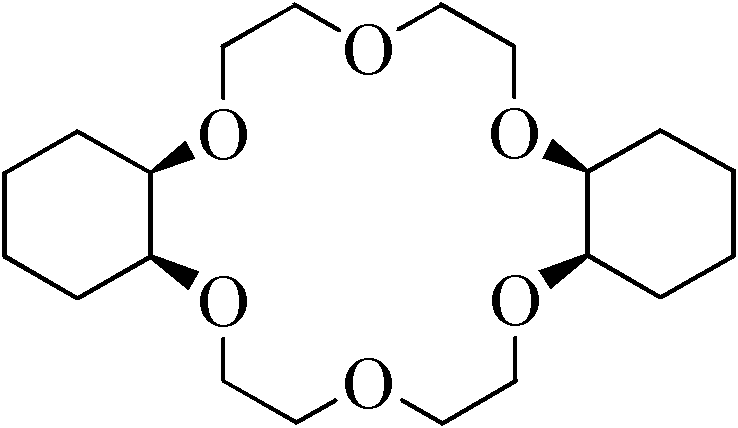
[0022] 1) Preparation of dicyclohexyl-18-crown-6
[0023] The reaction equation is as follows:
[0024]
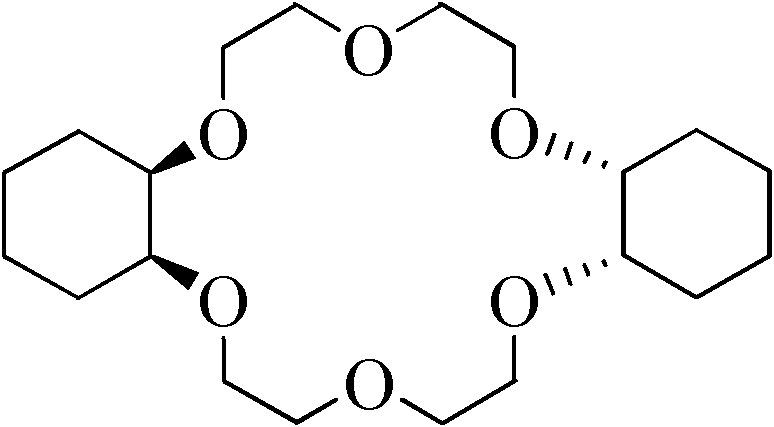
[0025] 2.0 g (0.0055 mol) of dibenzo-18-crown-6 was added to the autoclave. Add 0.28g (0.0027mol) of nano-metal ruthenium catalyst to 10ml of n-butanol, ultrasonically 30min to make it fully dispersed, then add it to the autoclave, and repeatedly rinse the beaker containing the catalyst with 40ml of n-butanol to fully add the catalyst into the kettle. After nitrogen replacement for 3 times and hydrogen replacement for 1 time, 10MPa hydrogen gas was charged for reaction. Control the reaction temperature to 100° C., and stop the reaction after 2 hours of reaction. After being cooled to room temperature, the mixture of the reaction product and the catalyst was centrifuged, and the product was detected by gas chromatography to show that the yield of dicyclohexyl-18-crown-6 was 91.2%, the cis-isomer content was 86.4%, and the trans-isomer content was 86.4%. The conformat...
Embodiment 2
[0029] 1) Add 20g (0.055mol) of dibenzo-18-crown-6 into the autoclave. Add 0.07g (0.0007mol) nano-metal ruthenium catalyst to 10ml of n-octanol, ultrasonic 30min to make it fully dispersed, then add it to the autoclave, rinse the beaker containing the catalyst repeatedly with 300ml of n-octanol, so that all the catalyst is added into the kettle. After nitrogen replacement for 3 times and hydrogen replacement for 1 time, 2MPa hydrogen gas was charged for reaction. Control the reaction temperature to 200° C., and stop the reaction after 24 hours of reaction. After being cooled to room temperature, the mixture of the reaction product and the catalyst was centrifuged, and the gas chromatography detection of the product showed that the yield of dicyclohexyl-18-crown-6 was 92.7%, the cis-isomer content was 81.5%, and the trans-isomer content was 81.5%. The conformation content was 18.5%.
[0030] 2) the preparation of the used nano ruthenium metal catalyst of above-mentioned reac...
Embodiment 3
[0033] 1) Add 2g (0.0055mol) of dibenzo-18-crown-6 into the autoclave. Add 0.05g (0.0005mol) of nano-metal ruthenium catalyst to 10ml of toluene, ultrasonic 30min to make it fully dispersed, then add it to the autoclave, rinse the beaker containing the catalyst repeatedly with 40ml of toluene, so that all the catalyst is added to the kettle. After nitrogen replacement for 3 times and hydrogen replacement for 1 time, 7MPa hydrogen gas was charged for reaction. The reaction temperature was controlled at 135° C., and the reaction was stopped after 7 hours of reaction. After being cooled to room temperature, the mixture of the reaction product and the catalyst was centrifuged, and the gas chromatography detection of the product showed that the yield of dicyclohexyl-18-crown-6 was 90.5%, the cis-isomer content was 87.6%, and the trans-isomer content was 87.6%. The conformation content was 12.4%.
[0034] 2) the preparation of the used nano ruthenium metal catalyst of above-mentio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com