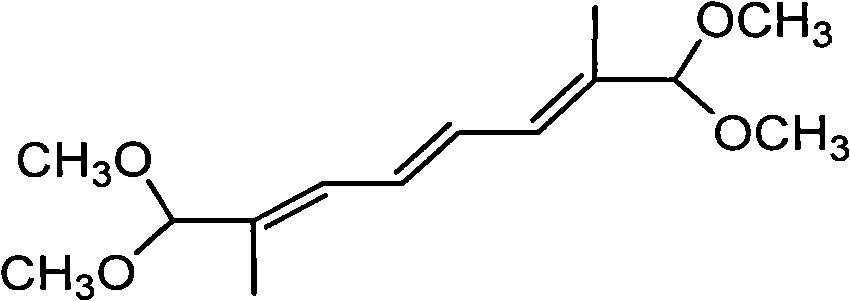
New synthesis process of 1,1,8,8-tetramethoxy-2,7-dimethyl-2,4,6-octatriene
A tetramethoxy, dimethyl technology, applied in the field of organic synthesis, can solve the problems of high cost, high cost, low yield and the like, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
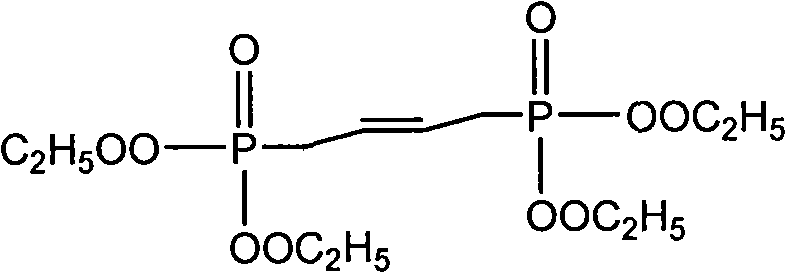
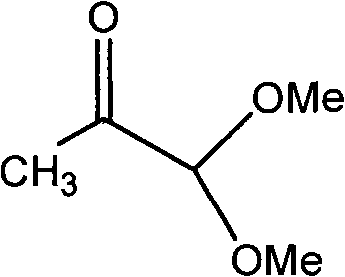
[0023] Take 32.8 grams (0.1 mole) of 1,4-diphosphate tetraethyl-2-butene and 23.6 grams (0.2 mole) of pyruvaldehyde dimethyl acetal into the reactor, add 1 liter of toluene and 1 liter of Cyclohexane, then add 32.8 grams of potassium carbonate, and finally add 30 grams of basic alumina with 12 grams of sodium hydroxide and 0.01 grams of 18-crown-6. Stir at room temperature for 4 hours. After the reaction is over Wash with 1 liter of water, divide the organic layer, dry with anhydrous sodium sulfate, filter, evaporate the solvent, and then evaporate the residual solvent and unreacted raw materials under a high vacuum of 0.20mmHg. The final 232 grams of product is analyzed by proton NMR Purity, the yield is 90%.
example 2
[0025] Take 32.8 g (0.1 mole) of 1,4-diphosphate tetraethyl-2-butene and 23.6 g (0.2 mole) of pyruvaldehyde dimethyl acetal into the reactor, add 1.25 liters of toluene and 1.25 liters of Cyclohexane, then add 16.4 grams of potassium carbonate, and finally add 30 grams of basic alumina with 14 grams of sodium hydroxide and 0.02 grams of 18-crown-6. Stir at room temperature for 4 hours. The post-treatment is the same. In Example 1, 230 grams of product were obtained, and the yield was 89%.
example 3
[0027] Take 32.8 grams (0.1 mole) of 1,4-diphosphate tetraethyl-2-butene and 23.6 grams (0.2 mole) of pyruvaldehyde dimethyl acetal into the reactor, add 1 liter of toluene and 1 liter of Cyclohexane, then add 16.4 grams of sodium sulfate, and finally add 30 grams of basic alumina with 16.8 grams of potassium hydroxide and 0.02 grams of 12-crown-4. Stir at room temperature for 4 hours. The post-treatment is the same. In Example 1, 210 grams of product were obtained, and the yield was 81.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com