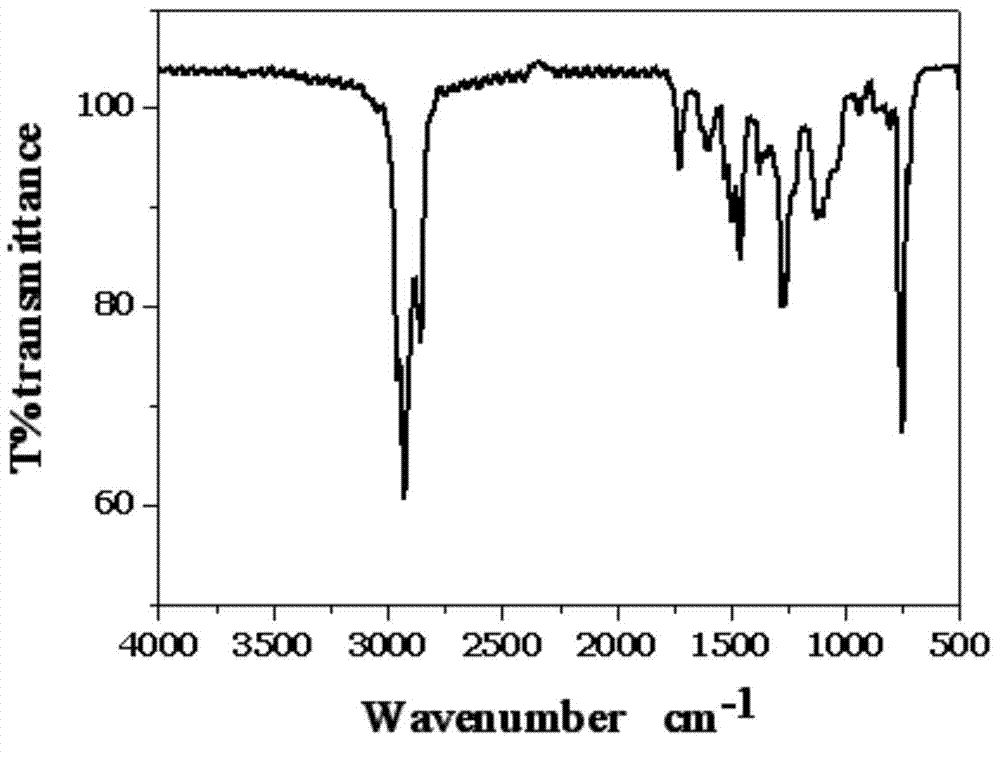
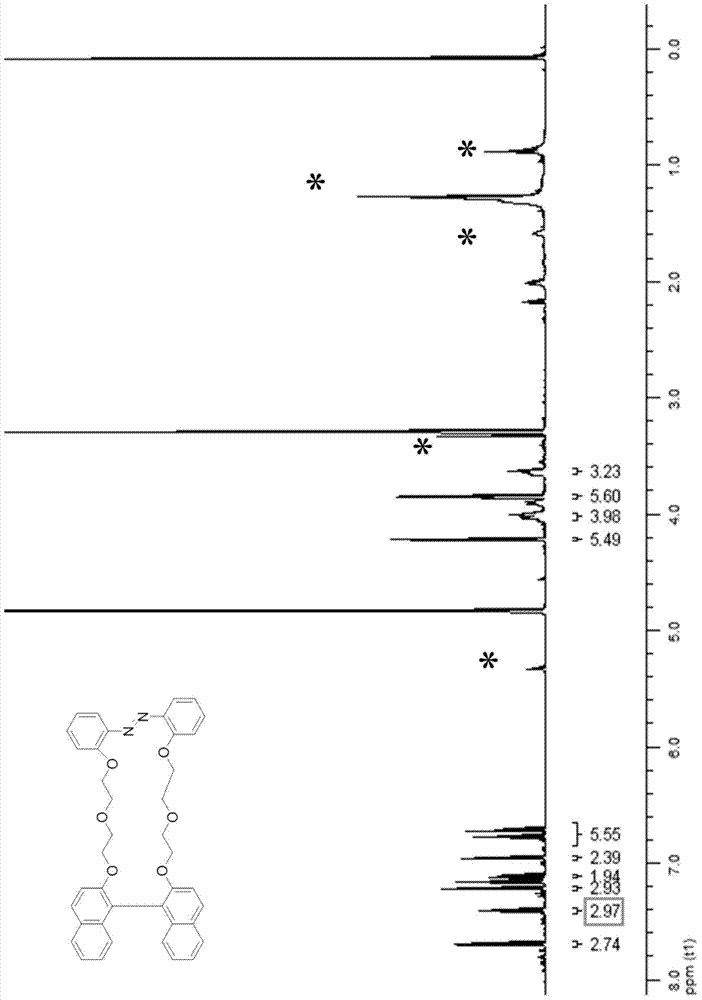
Photosensitive chiral macrocyclic molecule and preparation method and application thereof
A macrocyclic molecule, chiral technology, applied in the field of photoresponsive material preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of R-type binaphthyl photosensitive chiral macrocyclic molecule
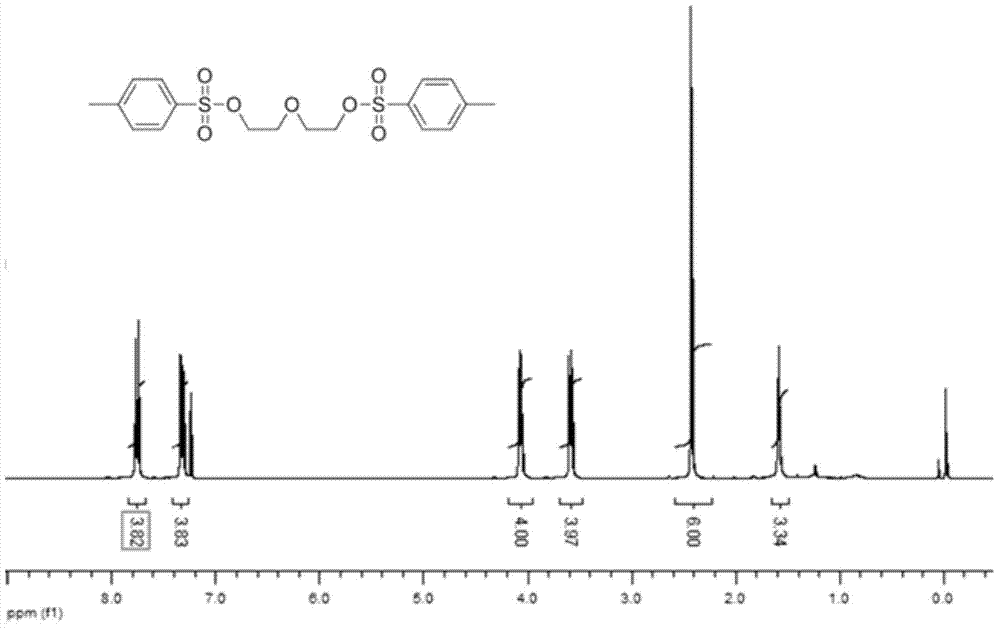
[0039] a). Dissolve 5.000g (0.0472mol) of diethylene glycol, 4.774g (0.1038mol) of sodium hydroxide and 19.72g (0.1038mol) of p-toluenesulfonyl chloride in 40ml tetrahydrofuran under the protection of nitrogen, and stir at room temperature for 10h , the product was recrystallized in distilled water, washed and dried to obtain white solid powder Intermediate 1, its structural formula is as follows:
[0040]
[0041] NMR spectrum such as Figure 7 , 1H NMR (300MHz, CDCl3) data: δ (ppm) 2.428 (s, 6H), 3.588 (t, J=4.8Hz, 4H), 4.035 (t, J=4.8Hz, 4H), 7.319 (d, J = 8.1 Hz, 4H), 7.774 (d, J = 8.1 Hz, 4H).
[0042]b). Take 5.8g (0.014mol) of the intermediate 1, 1g (0.0047mol) of o-dihydroxyazobenzene, 4.56g (0.014mol) of cesium carbonate and 0.504g (0.0014mol) of dibenzo-octadecacrown Dissolve in 50ml of N,N-dimethylformamide, stir at 80°C for 13h under nitrogen atmosphere, add ethyl ...
Embodiment 2
[0049] Example 2: Photoresponsive complexation and release of chiral ammonium salts
[0050] Take 2 mg of the target product to form a concentration of 2×10 -5 mol / L tetrahydrofuran solution, the solution was treated in the dark at 55°C for 8 hours, exposed to 365nm light, and the circular dichroism spectrum and UV-visible spectrum of the substance were measured at different exposure times. image 3 The middle square line is the circular dichroism spectrum of the solution after long-time 365nm ultraviolet exposure, and the hollow triangle line is the circular dichroism spectrum of the solution after long-time 440nm exposure. changes happened, Figure 4 The UV-Vis spectrum graph lines under different illumination times have been marked in the figure. With the increase of the exposure time, the UV absorption at 365nm decreases, and the UV absorption at 440nm increases, indicating that the molecule has the property of light response. Add different concentrations of L-alanine et...
Embodiment 3
[0052] Example 3: Changes in Twisting Force Constant of Cholesteric Liquid Crystals
[0053] Take 0.1g of liquid crystal and add 0.002g of the target product, heat it to the clearing point of the liquid crystal on a hot stage, keep the temperature at a constant temperature for 1 hour, make it fully mixed, and then add it into a wedge-shaped liquid crystal box. The wedge-shaped liquid crystal cell was observed under a polarizing microscope, and the pitch length of the liquid crystal was measured to be 2065 μm. The liquid crystal device is alternately illuminated with 365nm and 440nm, and the measured pitch length is 1689μm and 2356μm. Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com