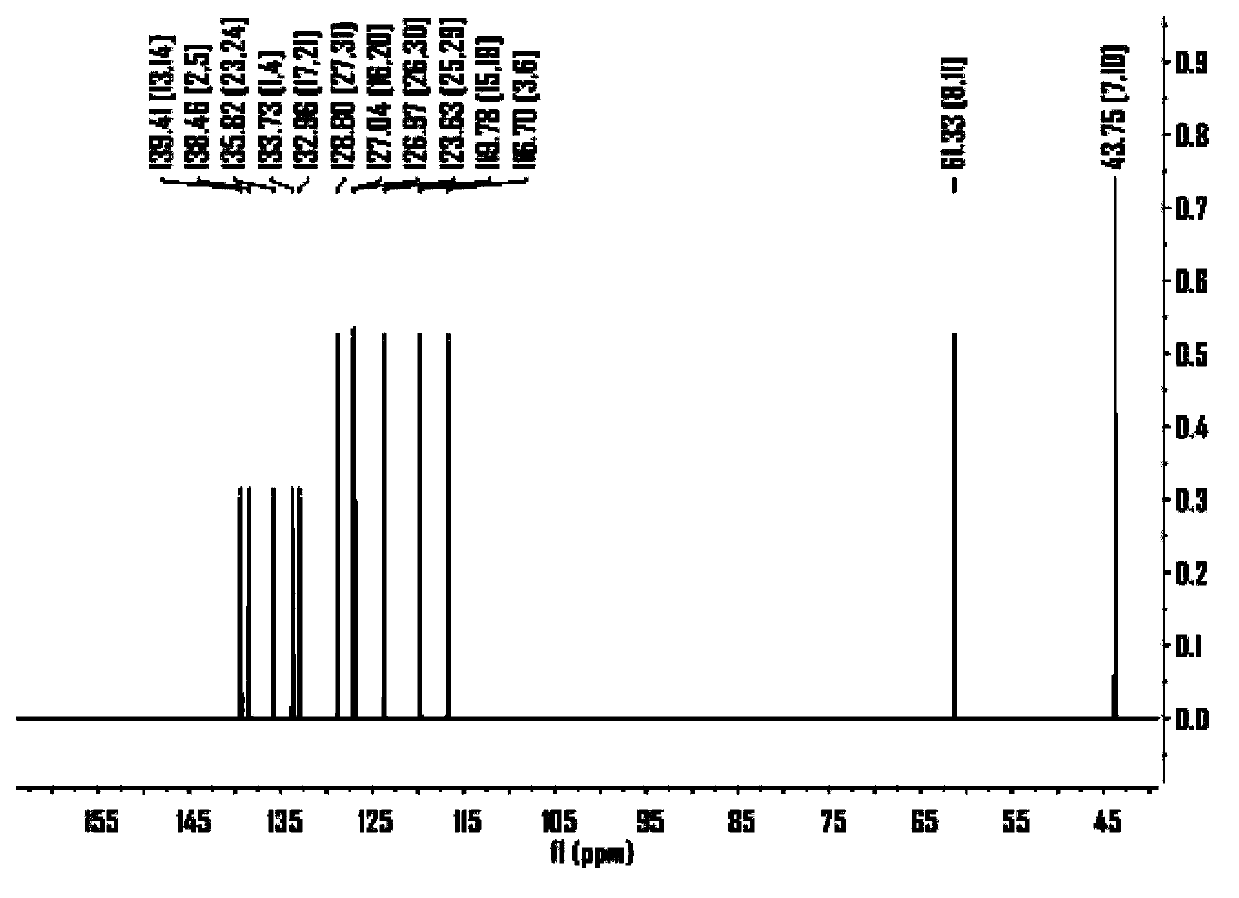
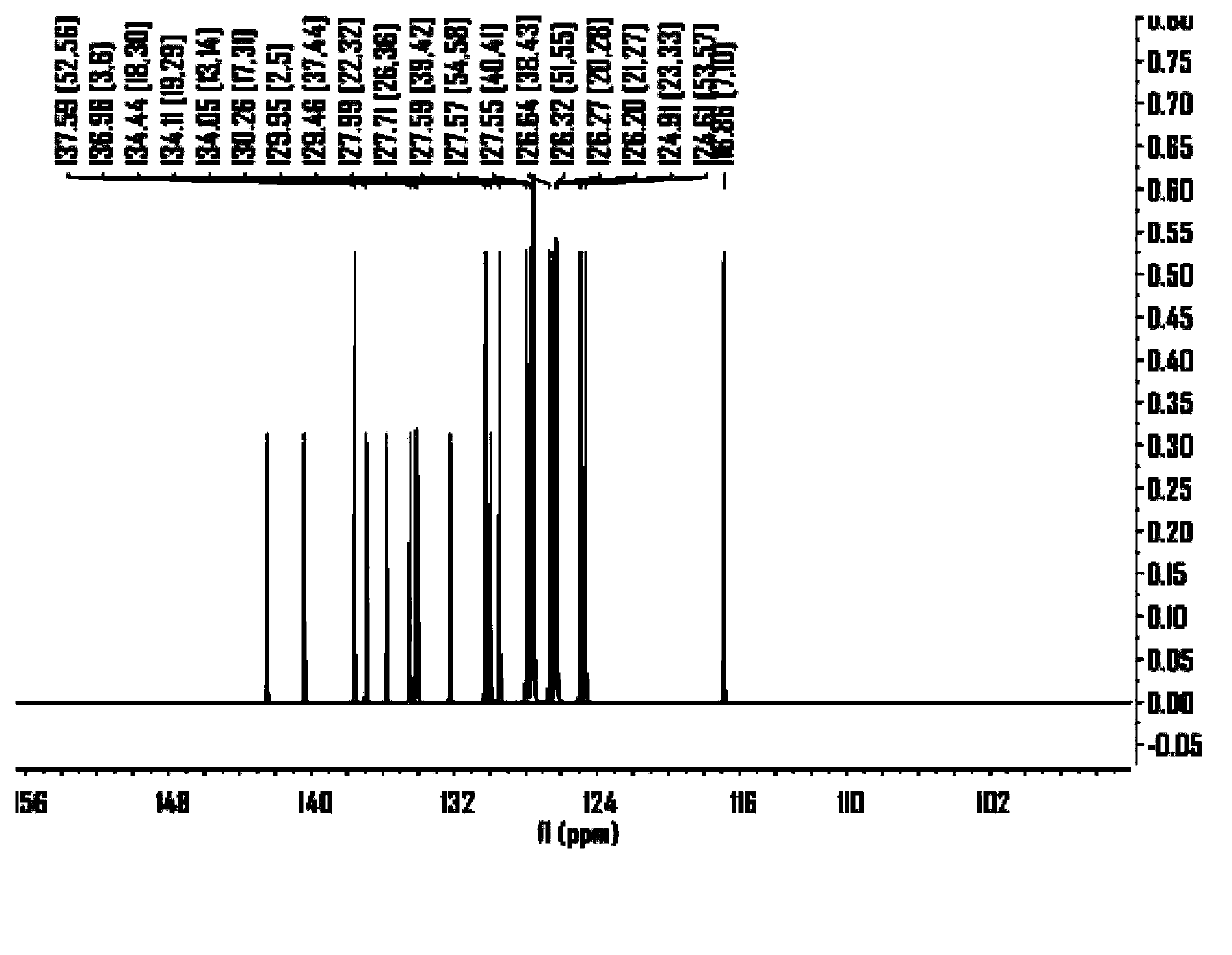
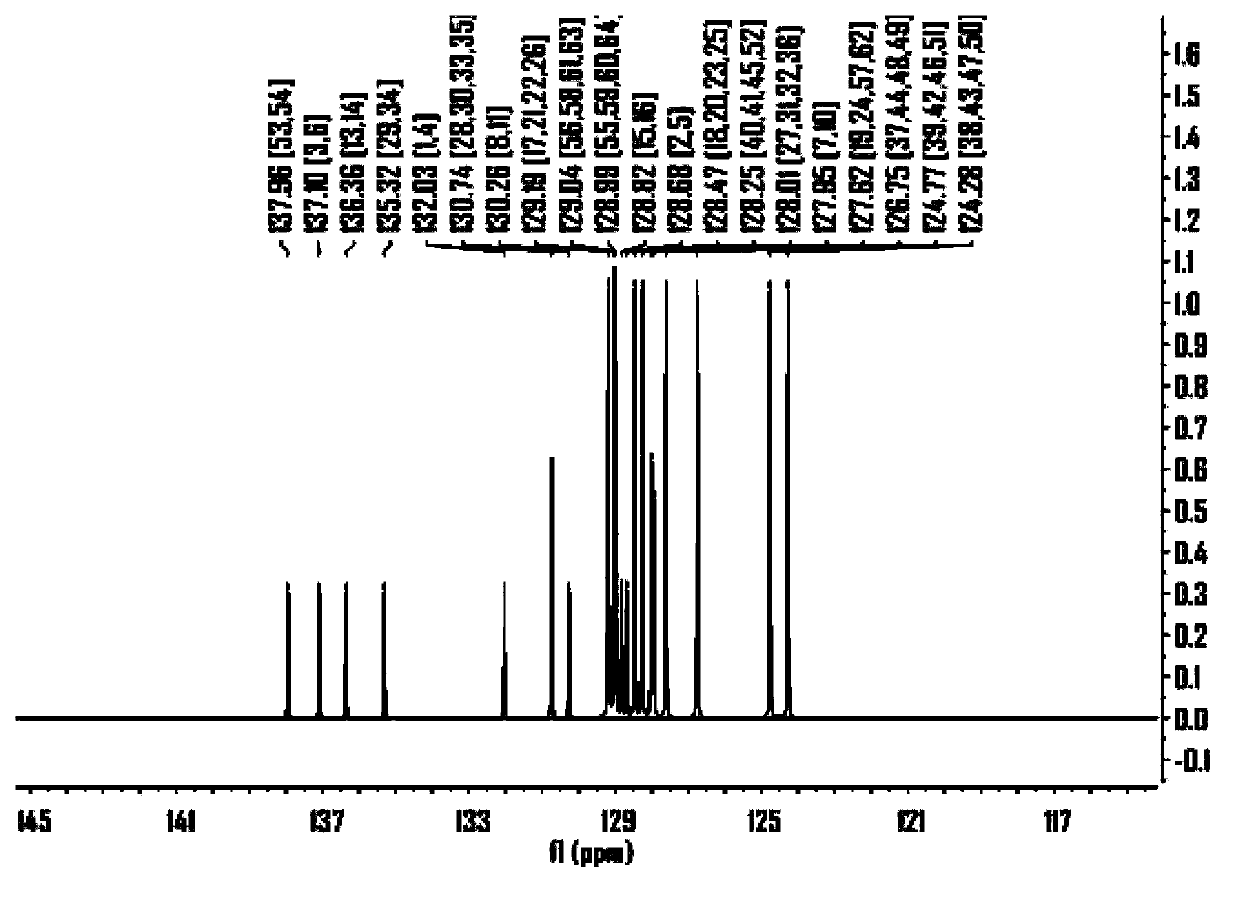
Benzodithiophene derivative organic electroluminescent material and application thereof
A technology of benzodithiophene and derivatives, applied in the field of organic electroluminescent display technology, the field of organic electroluminescent device compounds and its preparation, can solve the problem of low hole mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] The synthesis of embodiment 1 compound 1
[0125] (1) The first step
[0126]
[0127] Under the protection of Ar gas, add 10g (molecular weight 190, 0.0526mol) of BDT1, 250ml of dry THF into a dry reactor, cool to -80°C, add dropwise 53ml of t-BuLi (concentration 2.4M, 0.127mol), naturally heated to -10°C with stirring, then cooled to -50°C again, added dropwise 35ml (molecular weight 326, specific gravity 1.20, 0.129mol) of tributyltin chloride, and stirred overnight. The next day, the reaction was terminated with 200ml of sodium bicarbonate (concentration 0.1M), the product was extracted with dichloromethane, and the product was separated by column chromatography (eluent: petroleum ether / triethylamine=95 / 5) to obtain 32 g of Colored oily product (76% yield).
[0128] (2) The second step
[0129]
[0130] 9.16g of ditin compound synthesized in the first step (molecular weight 768, 0.01192mol) and 7.1g of 5-(thiophen-2-yl)-2-bromothiophene (molecular weight 24...
Embodiment 2
[0132] The synthesis of embodiment 2 compound 2
[0133]
[0134] The synthesis procedure is the same as the second step in Example 1, except that one of the raw materials, 5-(thiophen-2-yl)-2-bromothiophene, is changed to 5-(2-naphthyl)-2-bromothiophene, The product was obtained as a yellow solid.
[0135] Product MS (m / e): 606, elemental analysis (C 38 h 22 S 4 ): theoretical value C: 75.21%, H: 3.65%, S: 21.14%; measured value C: 60.23%, H: 2.75%, S: 37.02%.
Embodiment 3
[0136] The synthesis of embodiment 3 compound 3
[0137] (1) The first step
[0138]
[0139] Under the protection of Ar gas, add 10.3g of 2-bromonaphthalene (molecular weight 206, 0.05mol), 200ml of anhydrous THF into a 500mL three-necked flask, cool to -78°C, add dropwise 25ml (concentration 2.4M, 0.06mol ) of BuLi was added in 30 minutes, and stirred at -78°C for 30 minutes.
[0140] At -78°C, 5.4 g of BDTDO (molecular weight 220, 0.0245 mol) solid was added thereto, and 20 ml of THF was added. After the addition was completed, the mixture was warmed up to room temperature with natural stirring, and then stirred at room temperature for 2 h. Add 200ml of water and stir. Extract with ethyl acetate, and evaporate the ethyl acetate to dryness. Add 150ml acetic acid, 18gKI, 18g sodium hypophosphite to the solid. Heating to reflux under stirring, the solution quickly turned brownish-red, then brownish-yellow and turbid. After stirring for 1 hr, let it cool, filter to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com