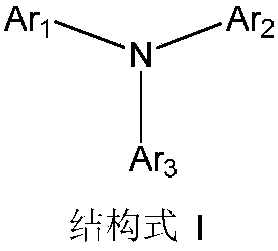
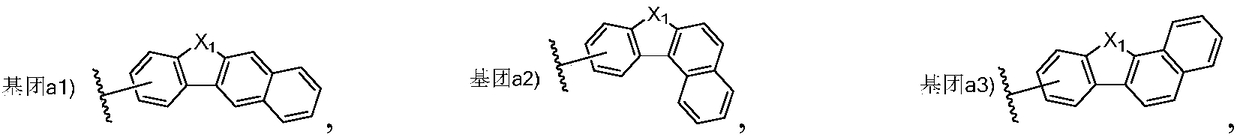
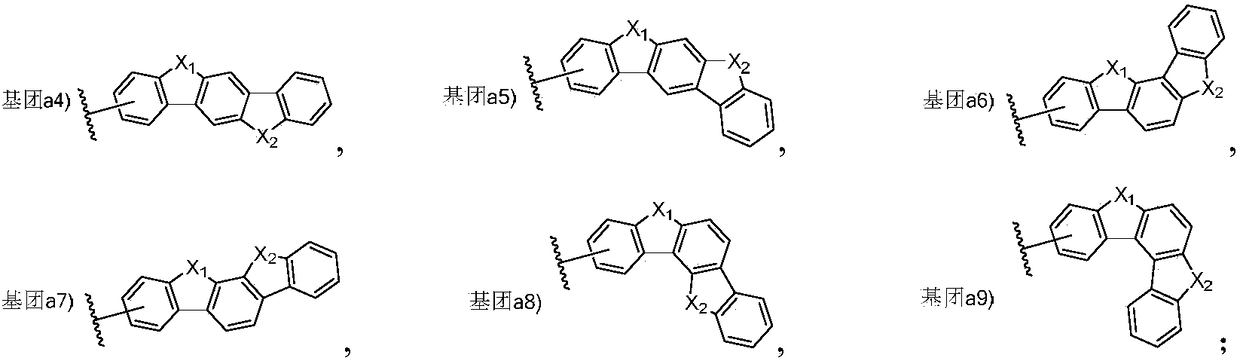
Triarylated amine derivative and organic electroluminescence device with same
A technology of derivatives and triarylamines, applied in the field of triarylamine derivatives and their organic electroluminescent devices, can solve the problems of unsatisfactory luminescence characteristics and achieve good hole transport performance, long service life and high luminescence Effect of Efficiency and Luminous Brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] (1) Preparation of Intermediate A
[0087]
[0088] Preparation of Intermediate A-1
[0089] Add compound I-1 (22.7g, 105mmol), compound a (30.0g, 100mmol), Pd(PPh 3 ) 4(2.31g, 2mmol), toluene (300ml), aqueous sodium carbonate solution (2M, 150ml), and stirred at reflux for 8 hours. After cooling the above reaction solution to room temperature, extract it with toluene, combine the organic phases, wash the organic phases with saturated brine, dry and concentrate the organic phases, and perform column chromatography with silica gel as the stationary phase to obtain intermediate A- 1 (26.1 g, 72%).
[0090] Preparation of Intermediate A-2
[0091] Compound A-1 (24.2g, 70mmol) and dry dichloromethane (200ml) were successively added into the flask, and the reaction system was cooled to 0°C. Join BBr 3 (22.0g, 88mmol), then stirred at room temperature for 24 hours. After the reaction, the solution was cooled to -78°C, carefully deactivated with methanol, and then de...
Embodiment 1
[0157] Preparation of compound TM1
[0158]
[0159] In the round bottom flask, add compound X-1 (9.1g, 37.5mmol), compound C (11.1g, 37.5mmol), t-BuONa (5.4g, 56.25mmol) and, Pd 2 (dba) 3 (0.686g, 0.75mmol) and sonicated toluene (200mL), followed by the addition of P(t-Bu) dissolved in toluene (3mL) 3 (0.36g, 1.8mmol), refluxed overnight under the protection of nitrogen, after the reaction solution was cooled to room temperature, treated with ethyl acetate and water, and the obtained organic layer was washed with MgSO 4 Drying, solvent removal under reduced pressure, obtain the crude product of intermediate Y-1, take silica gel as stationary phase, dichloromethane / hexane is eluent, crude product is carried out column chromatography, obtains intermediate Y-1 (11.2g ,65%);
[0160] Under nitrogen protection, intermediate Y-1 (11.5g, 25.0mmol), compound C (7.4g, 25mmol), t-BuONa (4.2g, 37.5mmol), Pd 2 (dba) 3 (0.28g, 0.5mmol), sonicated toluene (300ml) and P(t-Bu) dissol...
Embodiment 2
[0162]
[0163] In the round bottom flask, add compound X-2 (12.0g, 37.5mmol), compound A (11.1g, 37.5mmol), t-BuONa (5.4g, 56.25mmol) and, Pd 2 (dba) 3 (0.686g, 0.75mmol) and sonicated toluene (200mL), followed by the addition of P(t-Bu) dissolved in toluene (3mL) 3 (0.36g, 1.8mmol), refluxed overnight under the protection of nitrogen, after the reaction solution was cooled to room temperature, treated with ethyl acetate and water, and the obtained organic layer was washed with MgSO 4 Drying, solvent removal under reduced pressure, obtain the crude product of intermediate Y-2, take silica gel as stationary phase, dichloromethane / hexane is eluent, crude product is carried out column chromatography, obtains intermediate Y-2 (13.4g ,67%);
[0164] Under nitrogen protection, intermediate Y-2 (13.4g, 25.0mmol), compound A (7.4g, 25mmol), t-BuONa (4.2g, 37.5mmol), Pd 2 (dba) 3 (0.28g, 0.5mmol), sonicated toluene (300ml) and P(t-Bu) dissolved in toluene (2ml) 3 (0.24g, 1.2mm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com