Bay area cyclization synthesis method for perylene bisimide derivative
A technology of perylene bisimide and synthesis method, which is applied in the field of cyclization synthesis of perylene bisimide derivatives, can solve problems such as unsatisfactory yield, achieve good remodification ability, simple reaction steps, Amplify the effect of conjugated systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
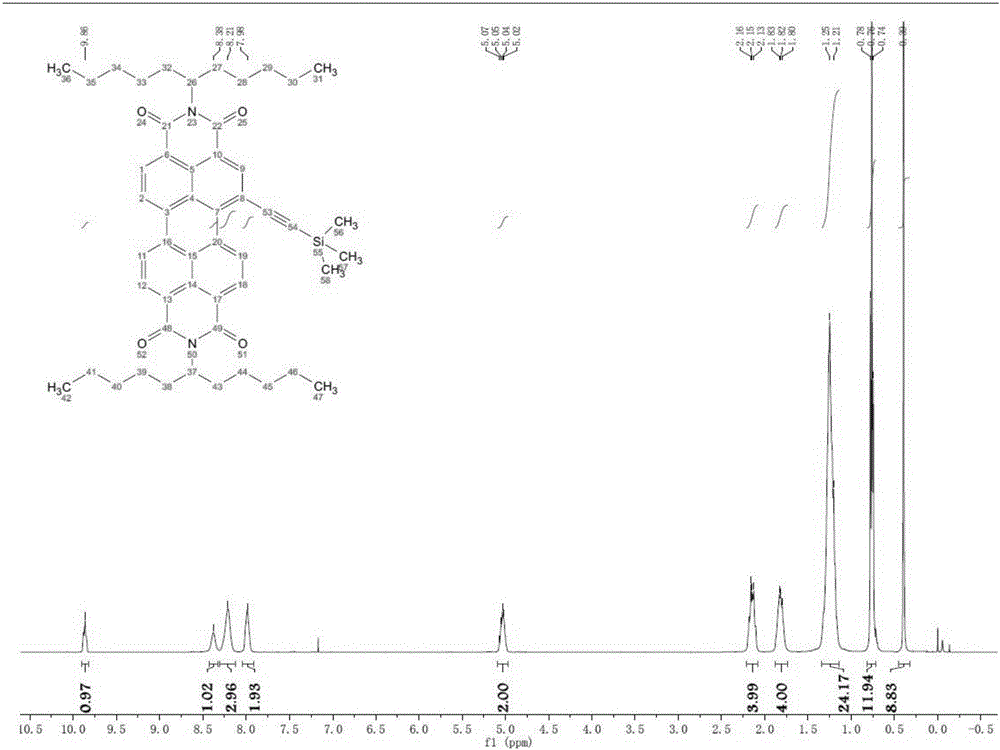
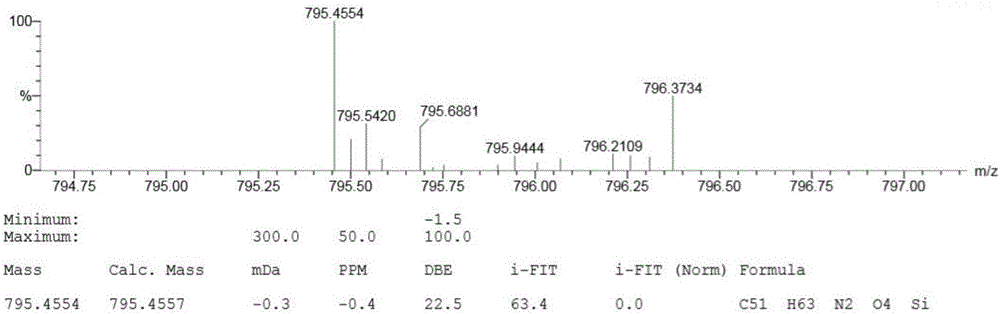
[0049] Dissolve 0.37 g of A in 200 ml of dichloromethane, add dropwise a dichloromethane solution containing 0.34 g of iodine monobromide under the protection of argon, and stir at -78°C for 1 hour. The reacted liquid was naturally warmed to room temperature, stirred for 3 hours, and then illuminated for 24 hours. After the reaction, quench the reaction with a dilute solution of sodium sulfite, dry with anhydrous magnesium sulfate, filter, remove the solvent, use 300-400 mesh silica gel column chromatography, and the eluent is petroleum ether: dichloromethane = 2:1, to obtain Orange-yellow solid B 0.31 g, yield 76%. The H NMR spectrum and mass spectrum of compound B are shown in image 3 and Figure 4 .
Embodiment 2
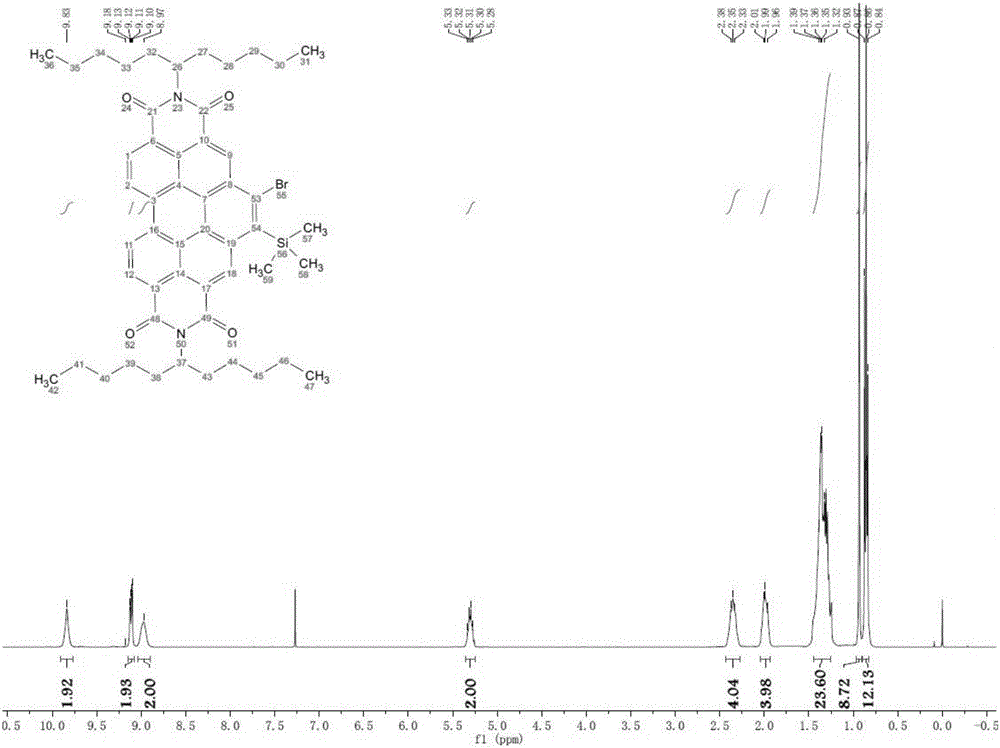
[0051]0.20 g of compound B was dissolved in 100 ml of dichloromethane, and under argon protection, a dichloromethane solution containing 0.13 g of iodine monochloride was added dropwise, and stirred at room temperature for 6 hours. After the reaction, quench the reaction with a dilute solution of sodium sulfite, dry with anhydrous magnesium sulfate, filter, remove the solvent, and use 300-400 mesh silica gel column chromatography, the eluent is petroleum ether: dichloromethane = 2:1, to obtain Orange-yellow solid C 0.18 g, yield 86%. The H NMR spectrum and mass spectrum of compound C are shown in Figure 5 and Figure 6 .
Embodiment 3
[0053] Add 0.15 g of compound C, 0.10 g of 3,4,5-trifluorophenylboronic acid, and 0.03 g of tetrakistriphenylphosphine palladium to a mixed solution of 5 ml of dilute potassium carbonate solution and 5 ml of tetrahydrofuran, under the protection of argon Heat to reflux and condense for 16 hours. After cooling to room temperature, extract three times with dichloromethane, dry the organic phase with anhydrous magnesium sulfate, remove the solvent, and use 300-400 mesh silica gel column chromatography, the eluent is petroleum ether: dichloromethane = 2:1, to obtain Orange-yellow solid D 0.15 g, yield 95%. The H NMR spectrum and mass spectrum of compound D are shown in Figure 7 and Figure 8 , whose UV-visible spectrum is shown in Figure 9 , the fluorescence spectrum see Figure 10 .
[0054] Referring to compounds with similar structures (see: Org.Lett., 2012, 14(17): 4654-4657), the total yield of the coupling step of this compound in the literature is less than 60%, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com