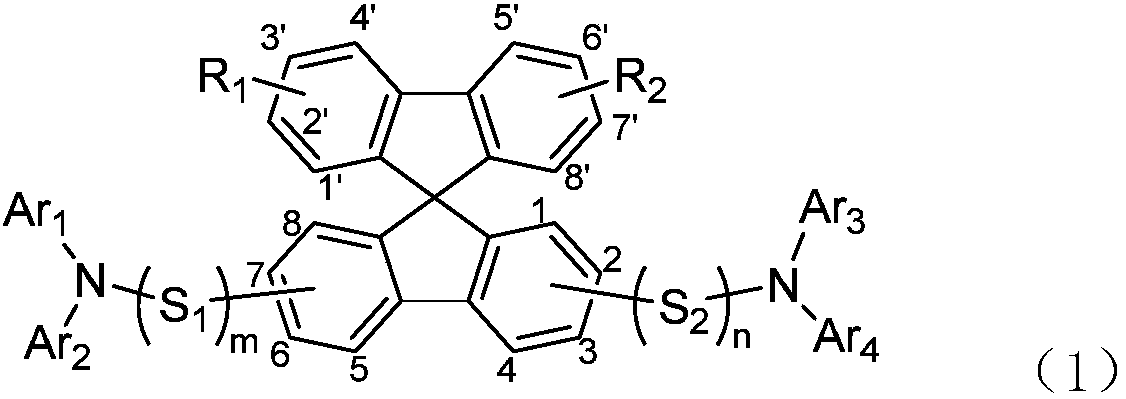
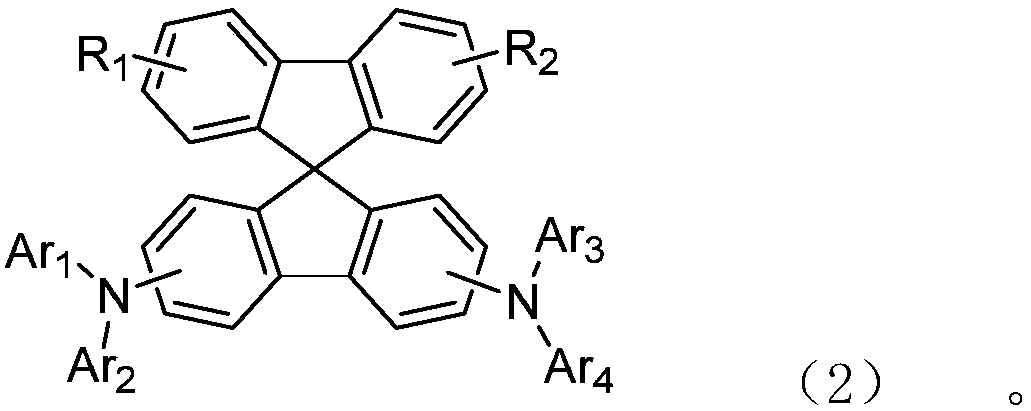
Diarylamine substituted spirobifluorene compound and application in OLED device
A technology of diarylamine and spirobifluorene, which is applied in the fields of organic chemistry and optoelectronic devices, and can solve problems such as performance differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment C1
[0064] (1) Example C1: Synthesis of 2-bromo-6-chloro-9,9'-spirobifluorene
[0065]
[0066] Fully dry the experimental device, add 35g of 2-bromo-1,1'-biphenyl (152mmol) and 400mL of dried tetrahydrofuran into a 1L four-necked flask under nitrogen, stir and dissolve, then cool down to below -78°C with liquid nitrogen , slowly add 61mL of 2.5M (152mmol) n-BuLi n-hexane solution dropwise; after the dropwise addition, stir at -78°C for 1h, then add 45g (152mmol) 2-bromo-6-chloro- 9-fluorenone was solid, after the addition was completed, it was kept at -78°C for 1h, and then stirred at room temperature for 12h. After the reaction was completed, 4M hydrochloric acid solution was added dropwise to quench the reaction, extracted with ethyl acetate, the organic phase was washed with saturated brine, and the solvent was spin-dried to obtain intermediate alcohol B1.
[0067] Without any purification, it was fed into a 1L dry three-neck flask, 160mL of acetic acid and 5g of 36% hydro...
Embodiment D1
[0079] (1) Example D1: Synthesis of N-([1,1'-biphenyl-4-yl)-6-chloro-N-(9-9-dimethyl-9-fluoren-2-yl)- 9,9'-spirobifluorenyl-2-amine]
[0080]
[0081] The experimental device was fully dried, and 19.3 g (45 mmol) of 2-bromo-6-chloro-9,9'-spirobifluorene and 17.9 g (49.5 mmol) of N-[1,1 '-biphenyl-4-yl]-9,9-dimethyl-9H-fluorene-2-amine, then add dry and degassed toluene as solvent, add 6.5g (67.5mmol) sodium tert-butoxide, 1.2g (2.25mmol) catalyst 1,1'-bis(diphenylphosphino)ferrocene, heated to 100-105°C, reacted for 16h. After the reaction was completed, it was cooled to room temperature, diluted with toluene, filtered through a pad of silica gel, and the filtrate was evaporated to remove the solvent in vacuo to obtain a crude product, which was purified by column chromatography to obtain 18.6 g of product D1 with a yield of 58%.
[0082] MS[M+H] + =710.23.
[0083] (2)D2-D10
[0084] Referring to the preparation method of intermediate D1, intermediates D2-D10 were syn...
Embodiment 1
[0118] refer to figure 1 The structure shown is to manufacture an OLED device. The specific steps are: the glass substrate (Corning glass 50mm*50mm*0.7mm) coated with ITO (indium tin oxide) with a thickness of 130nm is ultrasonically washed for 5 minutes with isopropanol and pure water respectively, Then use ultraviolet ozone to clean, and then the glass substrate is transferred to the vacuum deposition chamber; the hole injection material HAT-CN is vacuumed with a thickness of 5 nm (about 10 -7 Torr) is thermally deposited on the transparent ITO electrode, thereby forming a hole injection layer; on the hole injection layer, the compound 1-192 synthesized in the above-mentioned preparation example with a thickness of 110nm is vacuum-deposited to form a hole transport layer; HT2 is vacuum-deposited with a thickness of 20nm on the transport layer to form an electron blocking layer; as a light-emitting layer, the vacuum-deposited host EB and 4% guest dopant BD have a thickness of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com