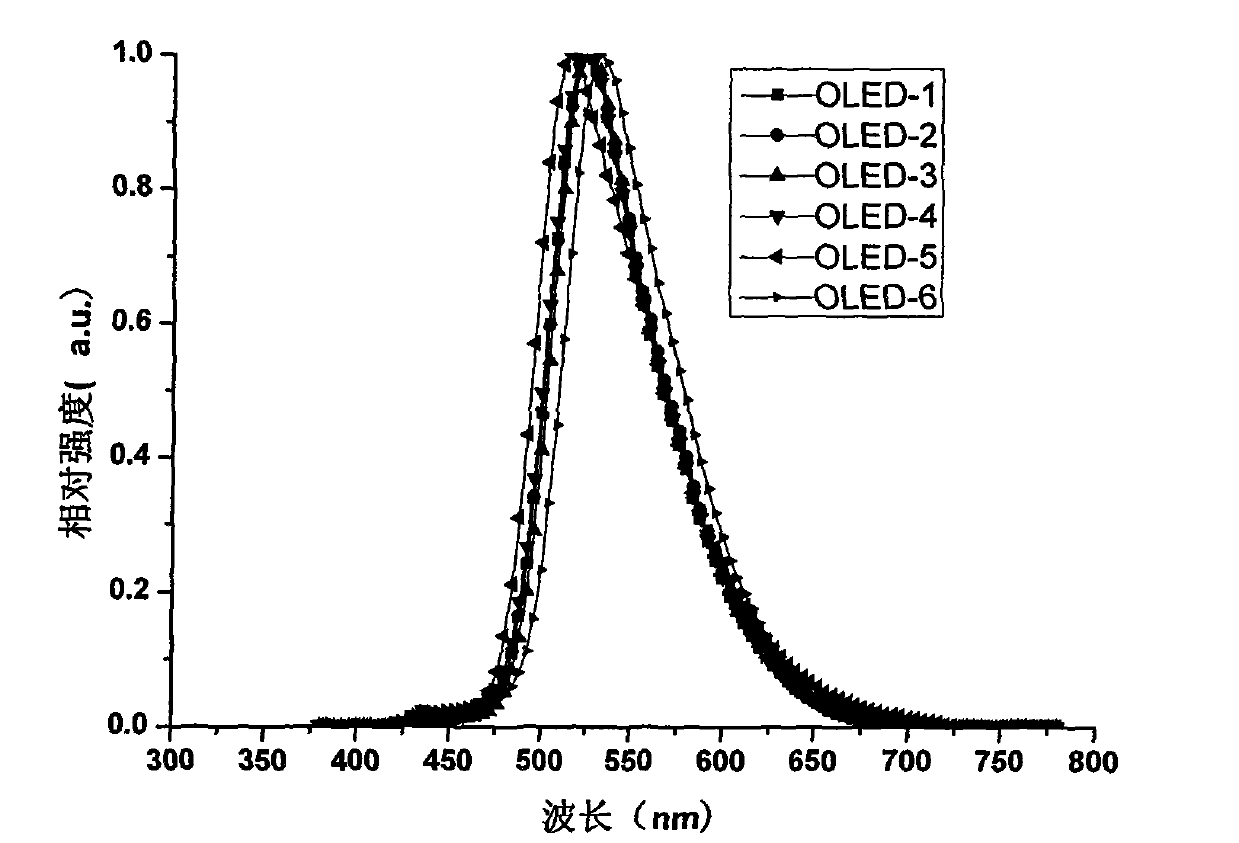
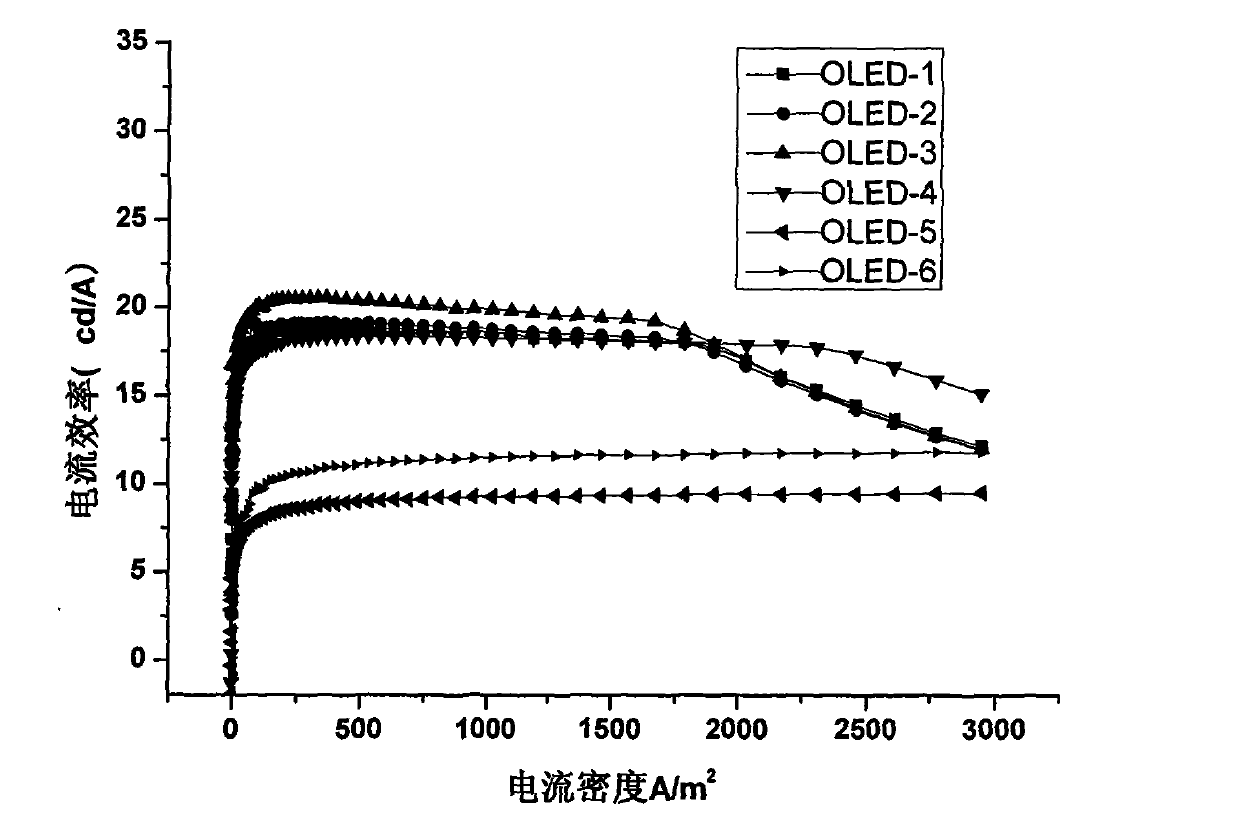
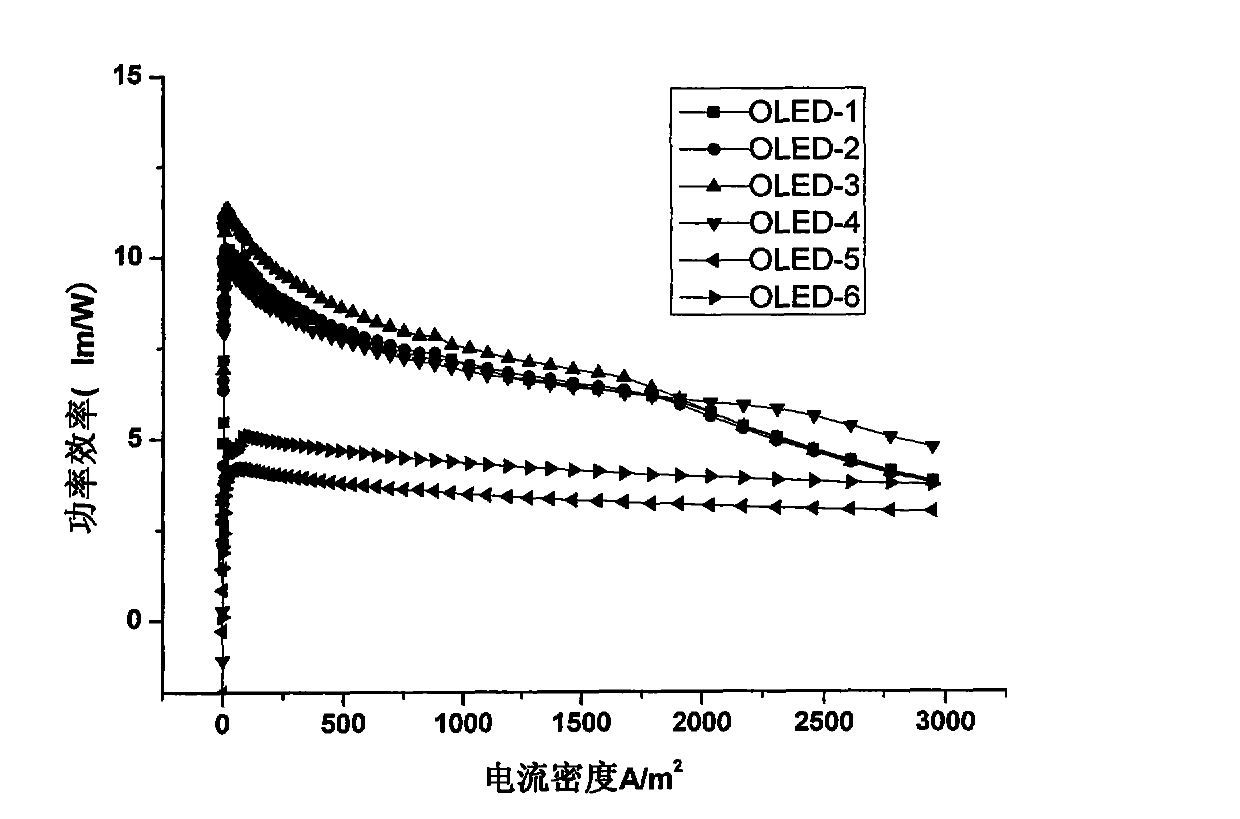
Organic electroluminescent material and application thereof
A carbon atom, selected technology, applied in luminescent materials, organic chemistry, circuits, etc., can solve the problems of difficulty in implementation, inability to make proper evaluation of compound performance, and lack of data on light-emitting devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] Preparation of some intermediates:
[0091] (1) Preparation of 7-bromo-1,2-benzo[a]anthracene (M1)
[0092]
[0093] In a 1000ml three-necked flask, 35 grams (147.4mmol) of 1,2-benzo[a]anthracene was dissolved in 550ml of N,N-dimethylsulfoxide (DMF), and another 32.8 grams (184.4mmol) of N-bromo Succinimide (NBS) was dissolved in 140ml DMF. Under room temperature and magnetic stirring, the NBS solution was added dropwise into the reaction flask through a constant pressure dropping funnel, and the addition was completed in about 30 minutes. The reaction was continued for 2 hours, and the end point of the reaction was monitored by TLC. The reaction solution was transferred to a beaker, and under rapid stirring, 400ml of water was added dropwise. After fully stirring, the precipitated yellow precipitate was filtered by suction, rinsed with water and absolute ethanol successively, and dried to obtain 35.8 grams of light orange-yellow solid. The yield : 78%.
[0094] ...
Embodiment 1
[0160] The synthesis of embodiment one compound V3
[0161]
[0162] Under the protection of argon, add 1.22 g (5 mmol) M2, 1.44 g (15 mmol) sodium tert-butoxide, 2.22 g (12 mmol) 2,4-dimethylbromobenzene, 0.029 g (0.05 mmol) di (Dibenzylideneacetone)palladium(0)(Pd(dba) 2 ), 0.20 grams (0.1 mmol) of tri-tert-butylphosphine (10% mass toluene solution) and 30 ml of dry toluene, magnetically stirred and refluxed for 5 hours, and TLC monitored the reaction end point. Cool, wash with water, separate the liquids, evaporate the solution by rotary evaporation, separate and purify by silica gel column chromatography, and elute with ethyl acetate:petroleum ether=1:12 (volume ratio) to obtain 1.65 g of a yellow solid with a yield of 73%.
[0163] Product FD-MS (m / z): 451.0, corresponding to: C 34 h 29 N=451.6, elemental analysis: C 34 h 29 N, theoretical value: C, 90.43; H, 6.47; N, 3.10; experimental value: C, 90.38; H, 6.40; N, 3.11; prove that the compound is the target produ...
Embodiment 2
[0164] The synthesis of embodiment two compound V8
[0165]
[0166] Under the protection of argon, add 1.84 g (5 mmol) M1, 1.44 g (15 mmol) sodium tert-butoxide, 1.47 g (6 mmol) N-p-biphenylaniline (M11), 0.029 g (0.05 mmol) (Dibenzylideneacetone)palladium(0)(Pd(dba) 2 ), 0.20 grams (0.1 mmol) of tri-tert-butylphosphine (10% mass toluene solution) and 35 ml of dry toluene, magnetically stirred and refluxed for 2 hours, and TLC monitored the reaction end point. Cool, wash with water, separate the liquid, evaporate the solution by rotary evaporation, separate and purify by silica gel column chromatography, and elute with ethyl acetate:petroleum ether=1:15 (volume ratio) to obtain 2.12 g of a yellow solid, with a yield of 90%.
[0167] Product FD-MS (m / z): 471.1, corresponding to: C 36 h 25 N=471.59, elemental analysis: C 36 h 25 N, theoretical value: C, 91.69; H, 5.34; N, 2.97; experimental value: C, 91.60; H, 5.27; N, 2.91; prove that the compound is the target product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com