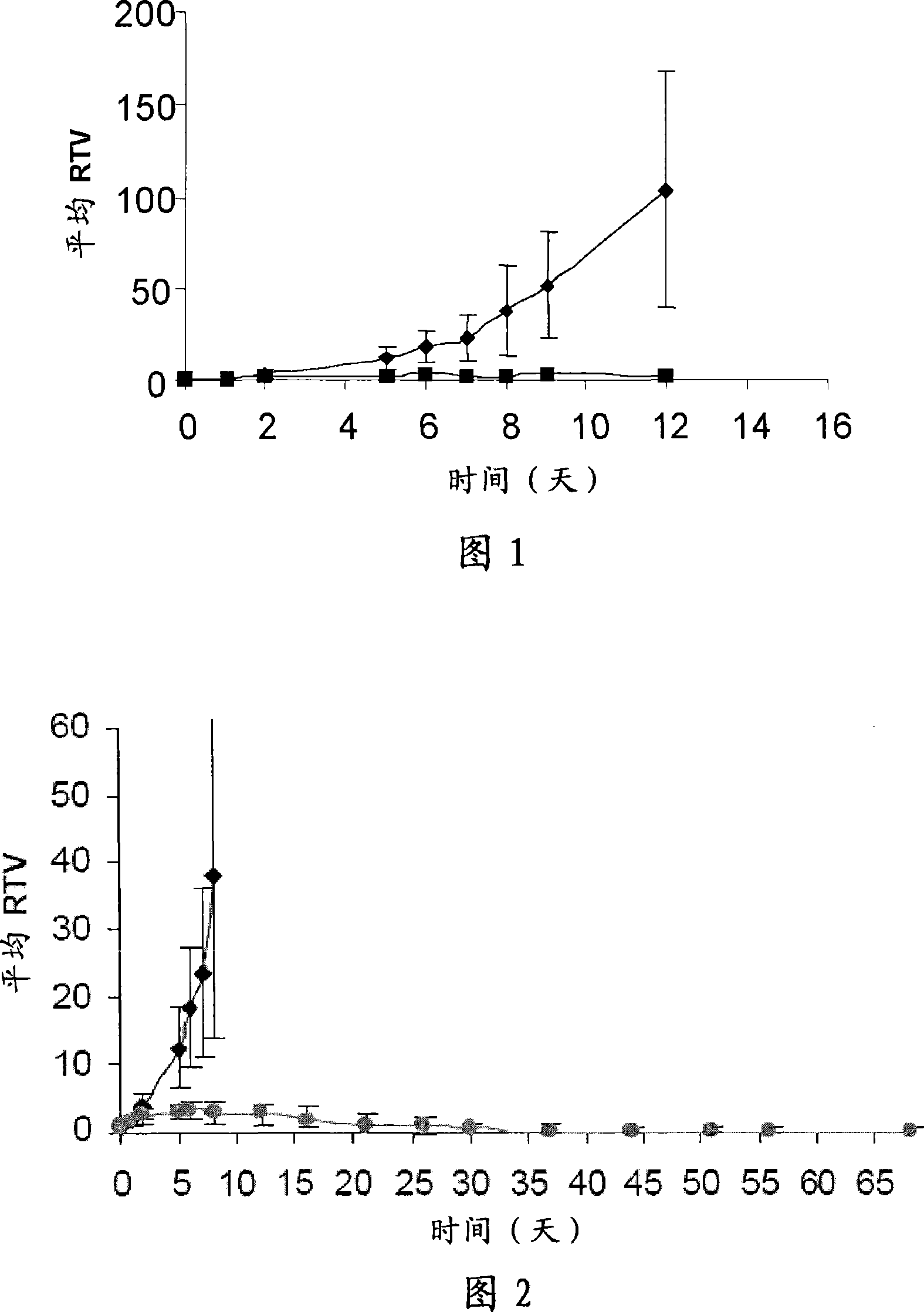
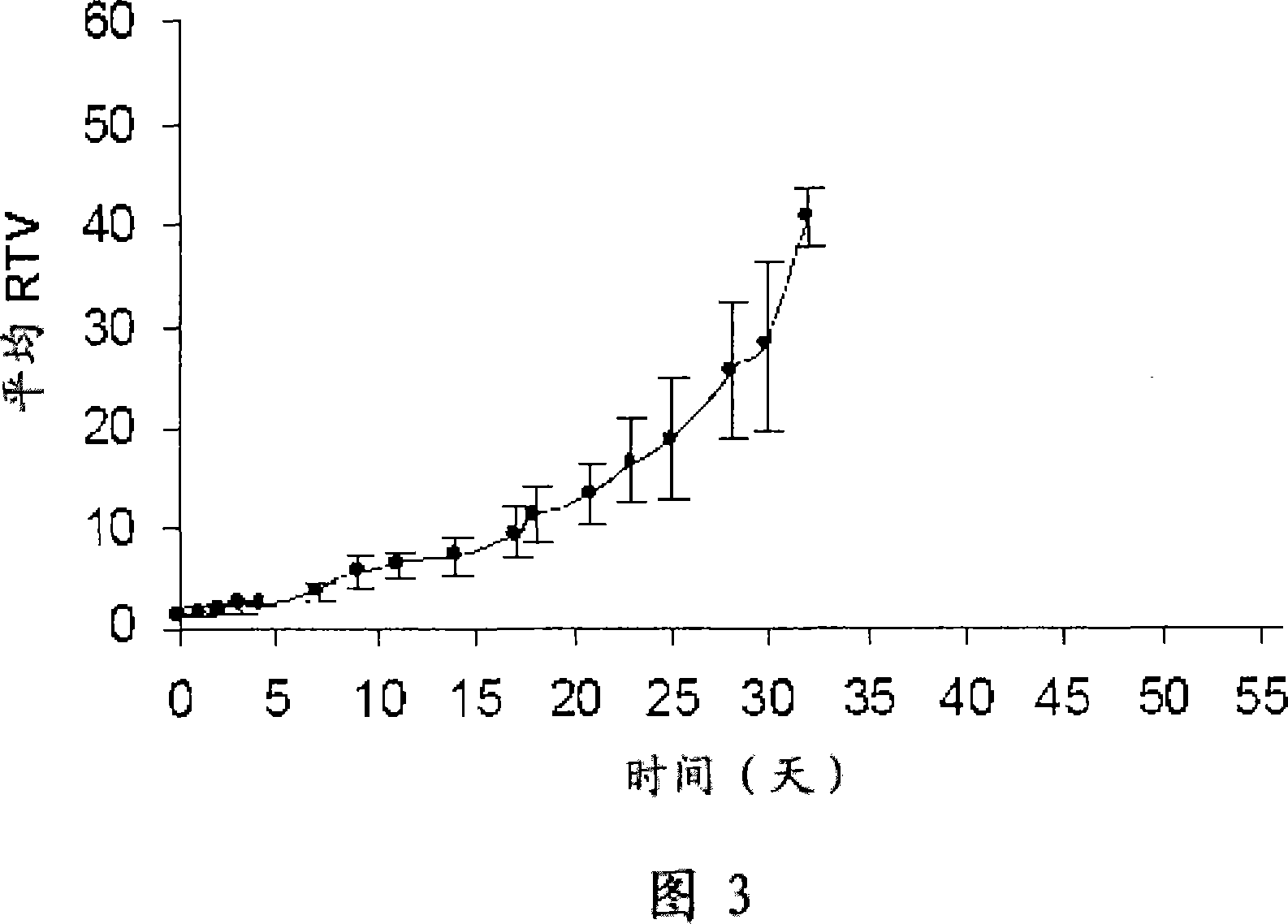
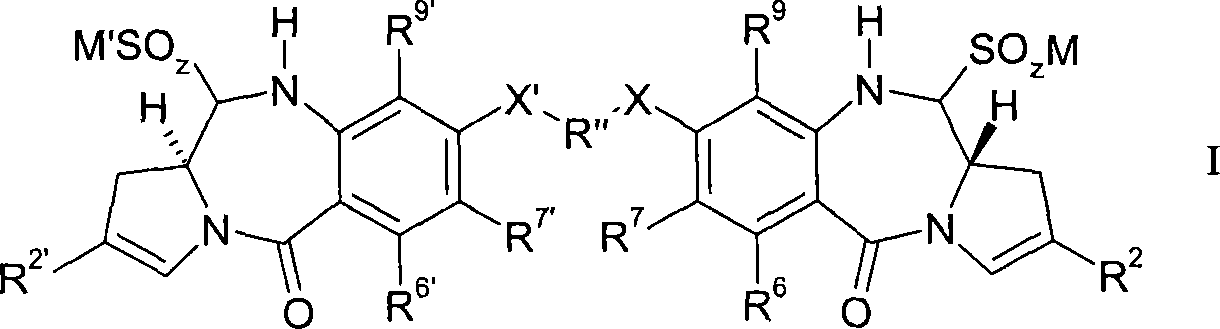
Pyrrolobenzodiazepines compound
A technology of compounds and solvates, applied in the field of pyrrolobenzodiazepine dimers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] (a) (2S, 4R)-N-(benzyloxycarbonyl)-2-tert-butyldimethylsilyloxymethyl-4-hydroxypyrrolidine (1)
[0268]
[0269] Starting from trans-4-hydroxy-L-proline, compound 1 was obtained in high yield according to a four-step process known in the art (S.J.Gregson et al., J.Med.Chem., 2004, 1161-1174 ).
[0270] (b) (2S, 4R)-N-(benzyloxycarbonyl-2-vin-butyldimethylsilyloxymethyl-4-oxyacetylpyrrolidine (2)
[0271]
[0272] To stirred 1(76.9g, 211mmol) in anhydrous THF (1L) solution. After stirring the reaction mixture for 16 hours, TLC (95:5 v / v CHCl 3 / MeOH) showed complete consumption of starting material. Excess solvent was removed by rotary evaporation, the residue was dissolved in EtOAc (1 L), washed with 1N HCl (2×1 L), H 2 O (1L), brine (1L), dried (MgSO 4 ). Filtration and evaporation of solvent gave acetate 2 (80.7 g, 94%) as a colorless oil: 1 H NMR (400MHz, CDCl 3 ) (rotamer) δ7.36-7.12 (m, 5H), 5.30-5.10 (m, 3H), 4.09-3.97 (m, 2H), 3.74-3.55 (m, 3H), 2....
Embodiment 2
[0313] (a) 1,1'-[[(propane-1,3-diyl)dioxy]bis[(11S,11aS)-10-(2,2,2-trichloroethoxycarbonyl)- 11-(tert-butyldimethylsilyloxy)-7-methoxy-2-(naphthalen-2-yl)-1,10,11,11a-tetrahydro-5H-pyrrolo[2 , 1-c][1,4]benzodiazepin-5-one]](17)
[0314]
[0315] At room temperature, TEA (0.15mL, 1.05mmol, 6.0eq) in H 2 A solution of O (1 mL) and EtOH (10 mL) was added to a solution of 13 (251 mg, 0.17 mmol) in toluene (6 mL). To this mixture was added 2-naphthylboronic acid (77.9mg, 0.45mmol, 2.6eq) and Pd(PPh 3 ) 4 (8.0 mg, 7 μmol, 0.04 eq). The reaction mixture was heated under microwave irradiation at 100 °C for 20 min, at which time thin layer chromatography (80:20 v / v EtOAc / Hexane) showed complete consumption of starting material. Excess solvent was removed, the residue was dissolved in EtOAc (20 mL), washed with H 2 O (15mL), brine (15mL) washed, dried (MgSO 4 ). Filtration and evaporation of the solvent gave the crude product, which was purified by flash column chromatography...
Embodiment 3
[0323] (a) 1,1'-[[(propane-1,3-diyl)dioxy]bis[(11S,11aS)-10-(2,2,2-trichloroethoxycarbonyl)- 11-(tert-butyldimethylsilyloxy)-7-methoxy-2-(thiophen-2-yl)-1,10,11,11a-tetrahydro-5H-pyrrolo[2 , 1-c][1,4]benzodiazepin-5-one]](20)
[0324]
[0325] The H of TEA (0.11mL, 0.82mmol, 6.0eq) 2 A solution of O (0.8 mL) and EtOH (5 mL) was added to a solution of 13 (197 mg, 0.14 mmol) in toluene (5 mL). To this mixture was added thiophene-2-boronic acid (45.5mg, 0.36mmol, 2.6eq) and Pd(PPh 3 ) 4 (6.3 mg, 5 μmol, 0.04 eq). The reaction mixture was stirred at room temperature for 3 hours at which time thin layer chromatography (80:20 v / v EtOAc / Hexanes) showed complete consumption of starting material. Excess solvent was removed, the residue was dissolved in EtOAc (20 mL), washed with H 2 O (15mL), brine (15mL) washed, dried (MgSO 4 ). Filtration and evaporation of the solvent gave the crude product, which was purified by flash column chromatography (80:20 v / v hexane / EtOAc) to aff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com