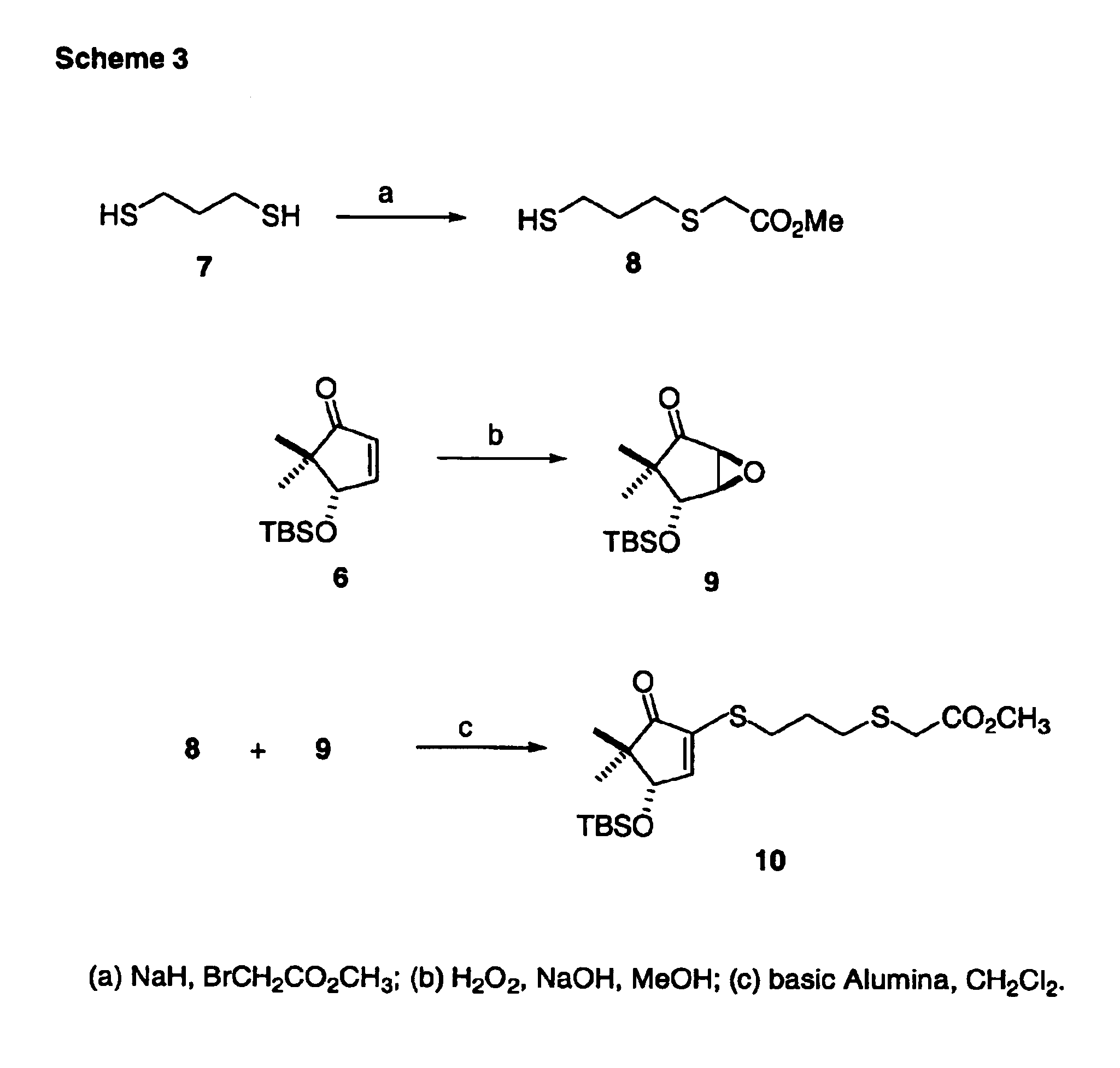
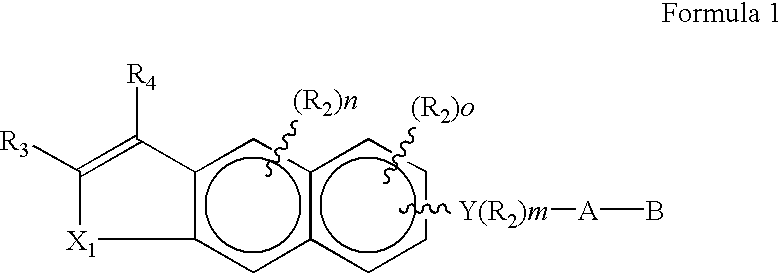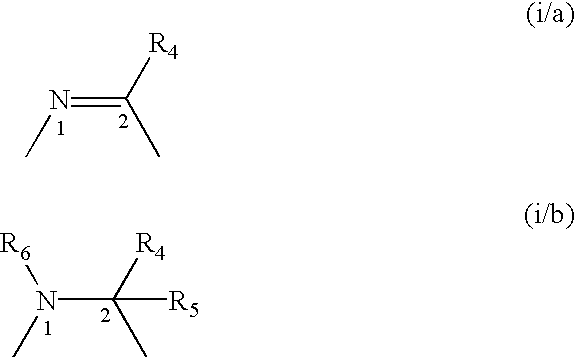Patents
Literature
593 results about "Triple bond" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
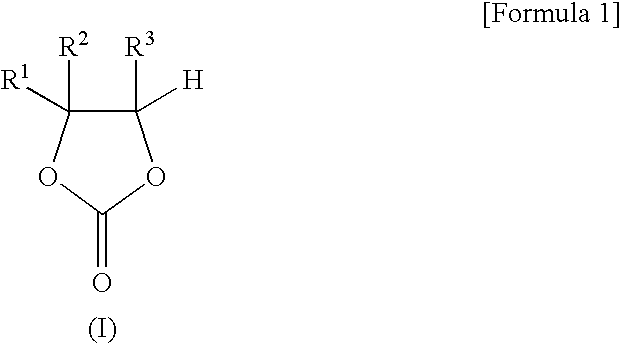
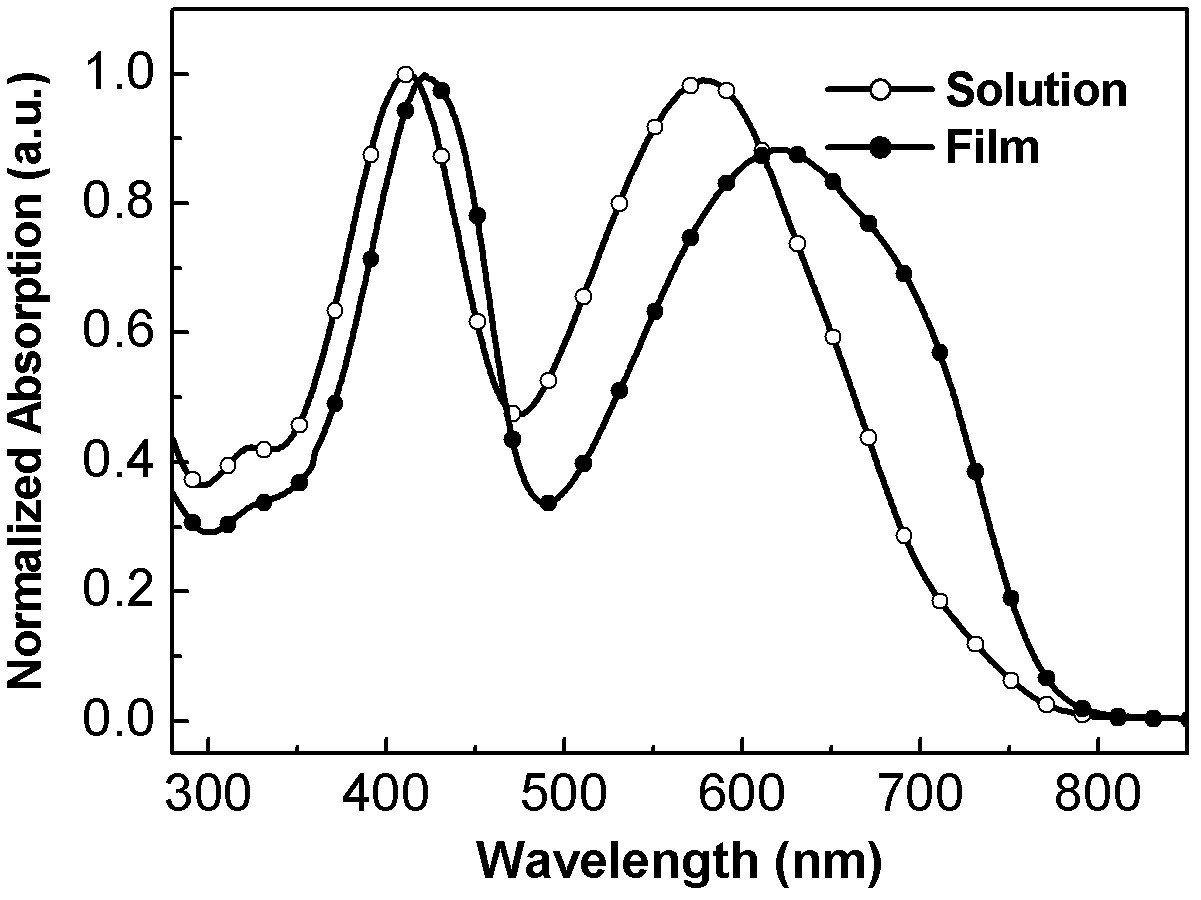
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as dinitrogen and carbon monoxide, are also triple bonded. In skeletal formula the triple bond is drawn as three parallel lines (≡) between the two connected atoms.
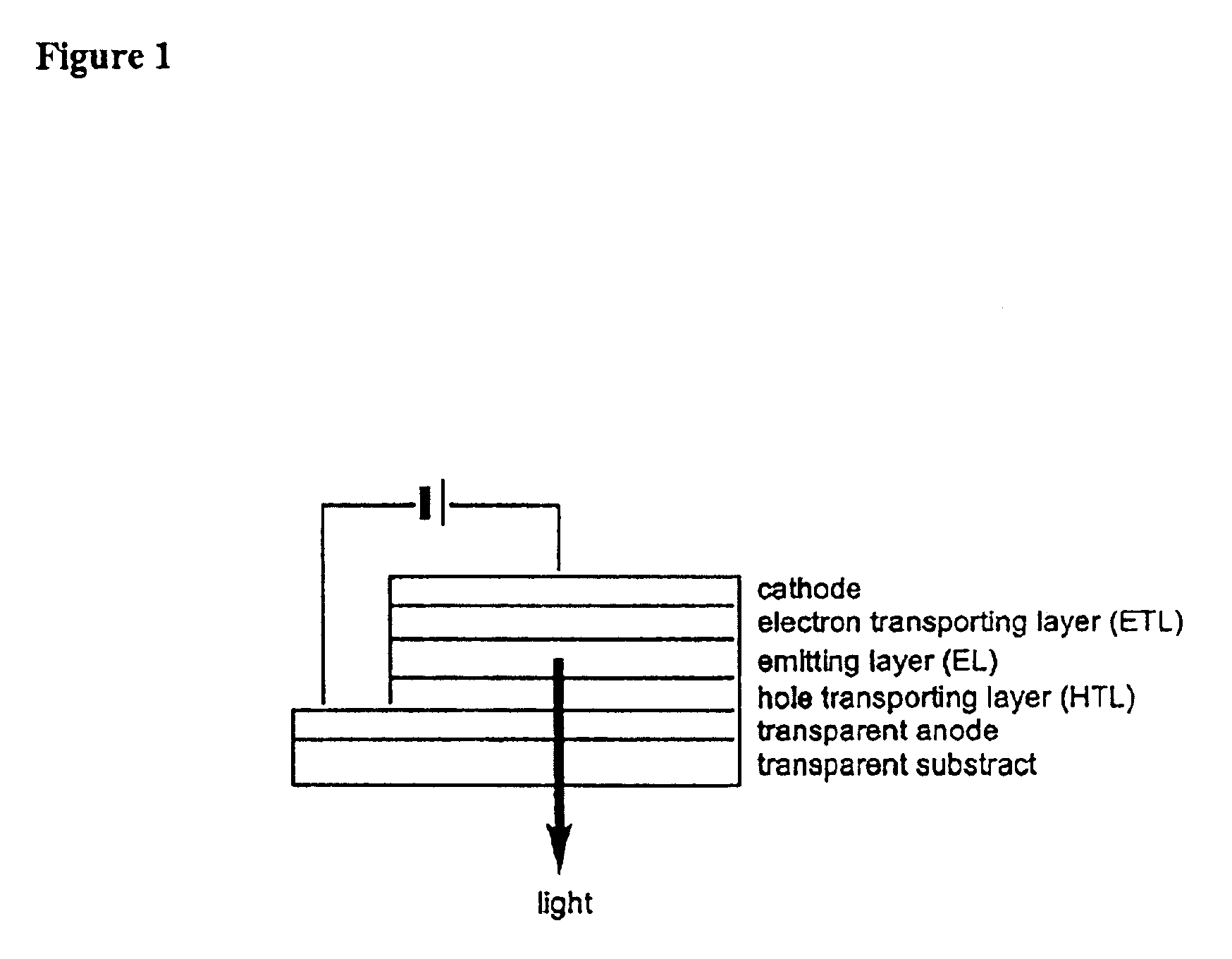
Organic electroluminescence device
InactiveUS6951693B2High efficiency of light emissionSolution to short lifeMethine/polymethine dyesOrganic chemistryAnthraceneHydrogen atom
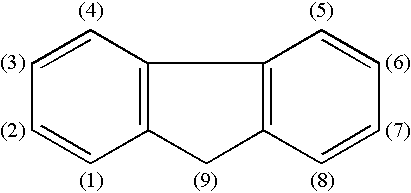
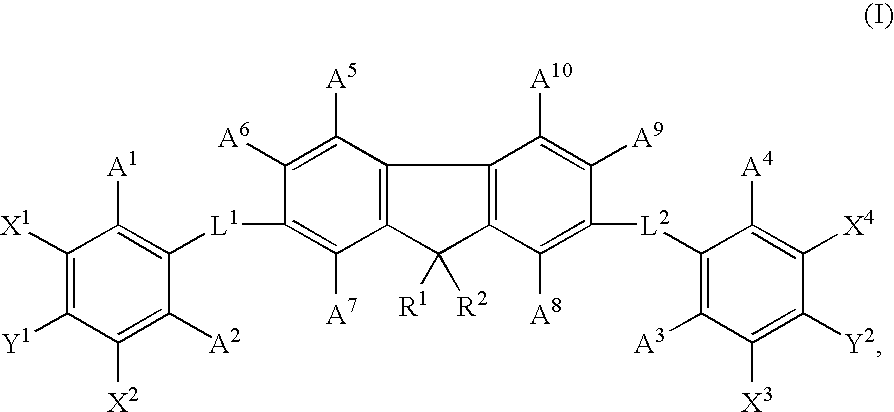
Materials for organic electroluminescence devices represented by following general formula [1]: wherein A represents a substituted or unsubstituted arylene group having 22 to 60 carbon atoms, X1 to X4 each independently represent a substituted or unsubstituted arylene group having 6 to 30 carbon atoms, X1 and X2 may be bonded to each other, X3 and X4 may be bonded to each other, Y1 to Y4 each independently represent an organic group represented by general formula [2], a to d each represent an integer of 0 to 2 and, when the arylene group represented by A has 26 or less carbon atoms, a+b+c+d>0 and the arylene group does not contain two or more anthracene nucleus;general formula [2] being: wherein R1 to R4 each independently represent hydrogen atom, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, a substituted or unsubstituted aryl group having 6 to 20 carbon atoms, cyano group or form a triple bond by a linkage of R1 and R2 or R3 and R4, Z represents a substituted or unsubstituted aryl group having 6 to 20 carbon atoms and n represents 0 or 1.
Owner:IDEMITSU KOSAN CO LTD
Organic electrolumescence device
InactiveUS20050038296A1High efficiency of light emissionSolution to short lifeOrganic chemistryMethine/polymethine dyesHydrogen atomOrganic group
Materials for organic electroluminescence devices are represented by following general formula [1]: general formula [1]wherein A represents a chrysene group X1 to X4 each independently represent a substituted or unsubstituted arylene group having 6 to 30 carbon atoms, X1 and X2 may be bonded to each other, X3 and X4 may be bonded to each other, Y1 to Y4 each independently represent an organic group represented by general formula [2], a to d each represent an integer of 0 to 2 and, a+b+c+d≧0; general formula [2] being: general formula [2]wherein R1 to R4 each independently represent hydrogen atom, a substituted or unsubstituted alkyl group having 1 to 20 carbon atoms, a substituted or unsubstituted aryl group having 6 to 20 carbon atoms, cyano group or form a triple bond by a linkage of R1 and R2 or R3 and R4, Z represents a substituted or unsubstituted aryl group having 6 to 20 carbon atoms and n represents 0 or 1.
Owner:IDEMITSU KOSAN CO LTD
Silicon carbide having low dielectric constant
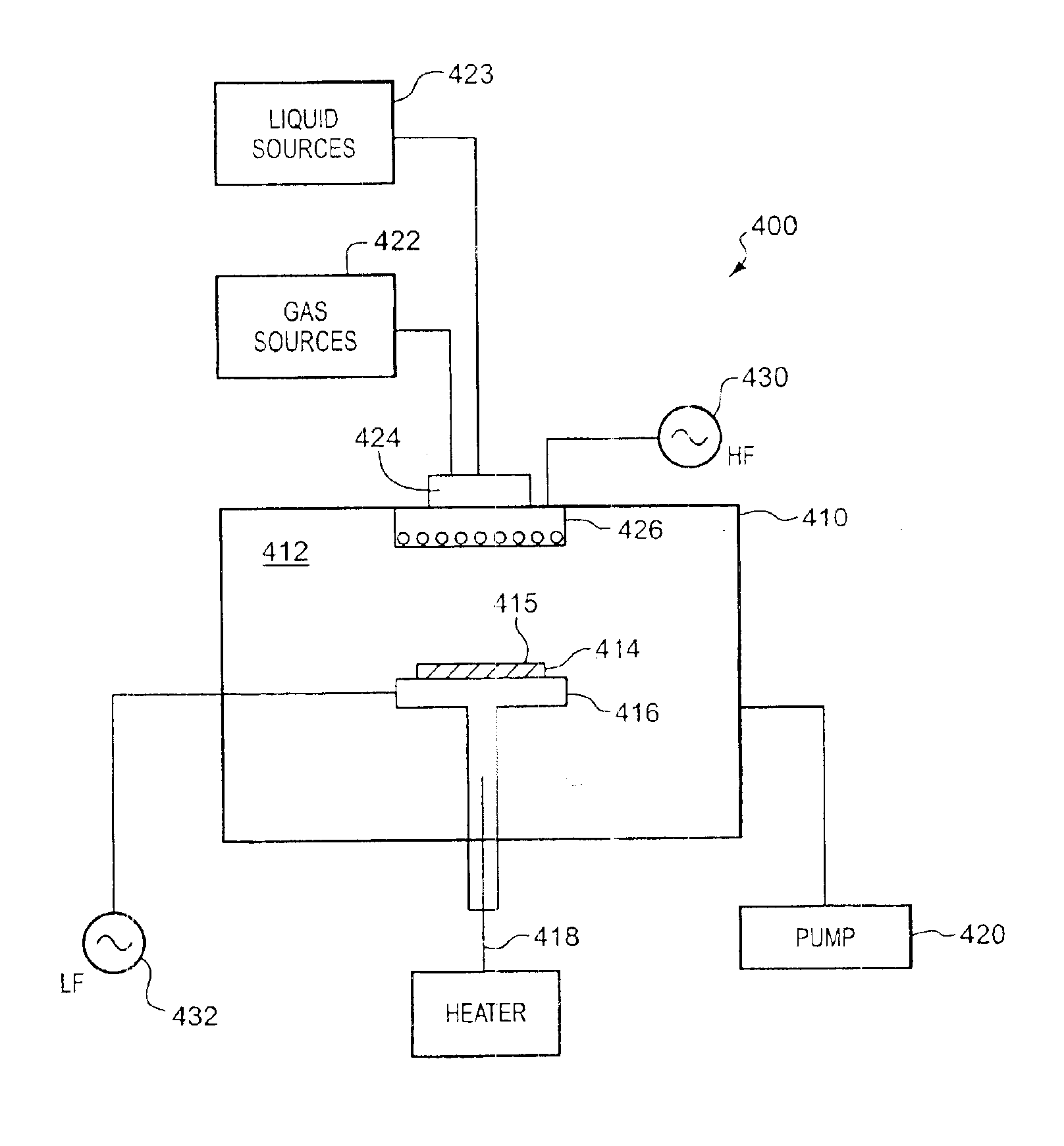
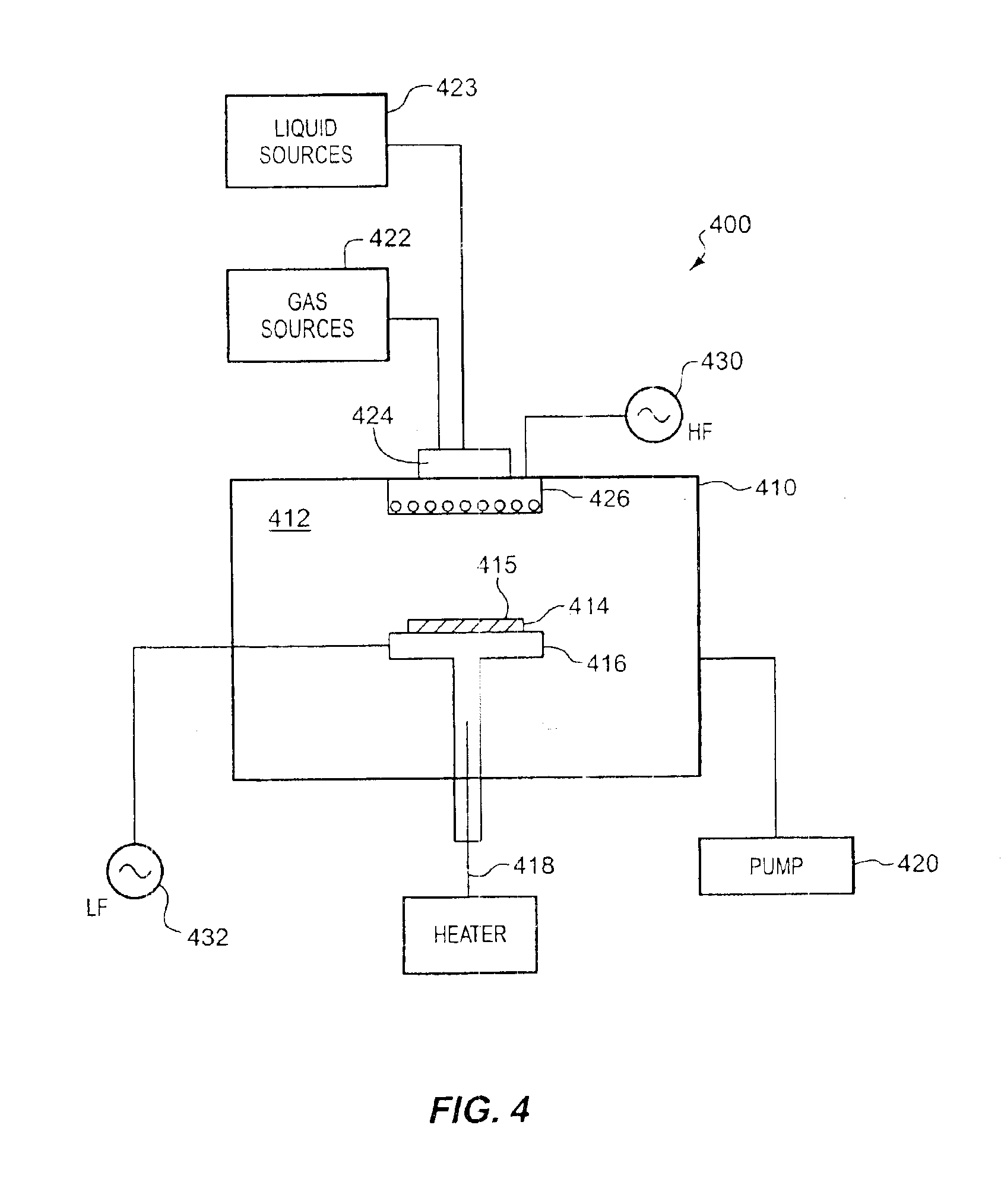
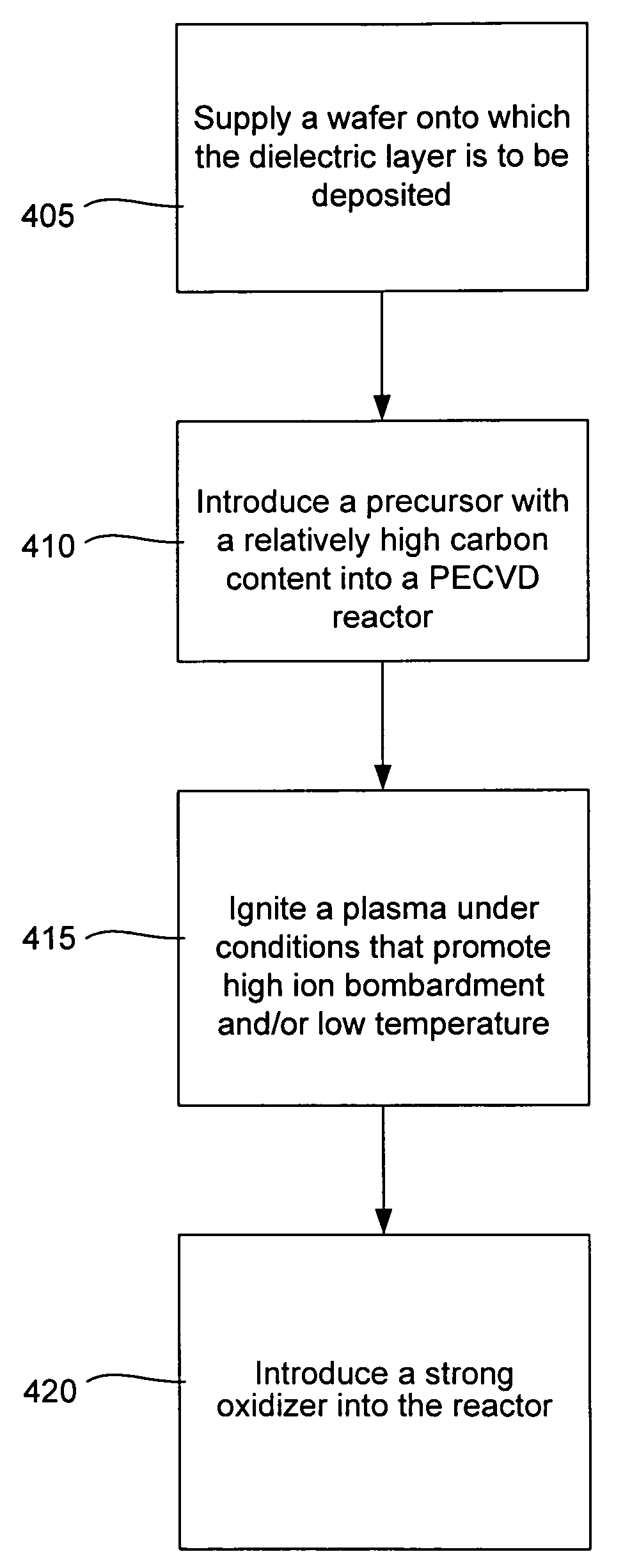
A low-k precursor reactant compound containing silicon and carbon atoms is flowed into a CVD reaction chamber. High-frequency radio-frequency power is applied to form a plasma. Preferably, the reaction chamber is part of a dual-frequency PECVD apparatus, and low-frequency radio-frequency power is applied to the reaction chamber. Reactive components formed in the plasma react to form low-dielectric-constant silicon carbide (SiC) on a substrate surface. A low-k precursor is characterized by one of: a silicon atom and a carbon—carbon triple bond; a silicon atom and a carbon—carbon double bond; a silicon—silicon bond; or a silicon atom and a tertiary carbon group.
Owner:NOVELLUS SYSTEMS
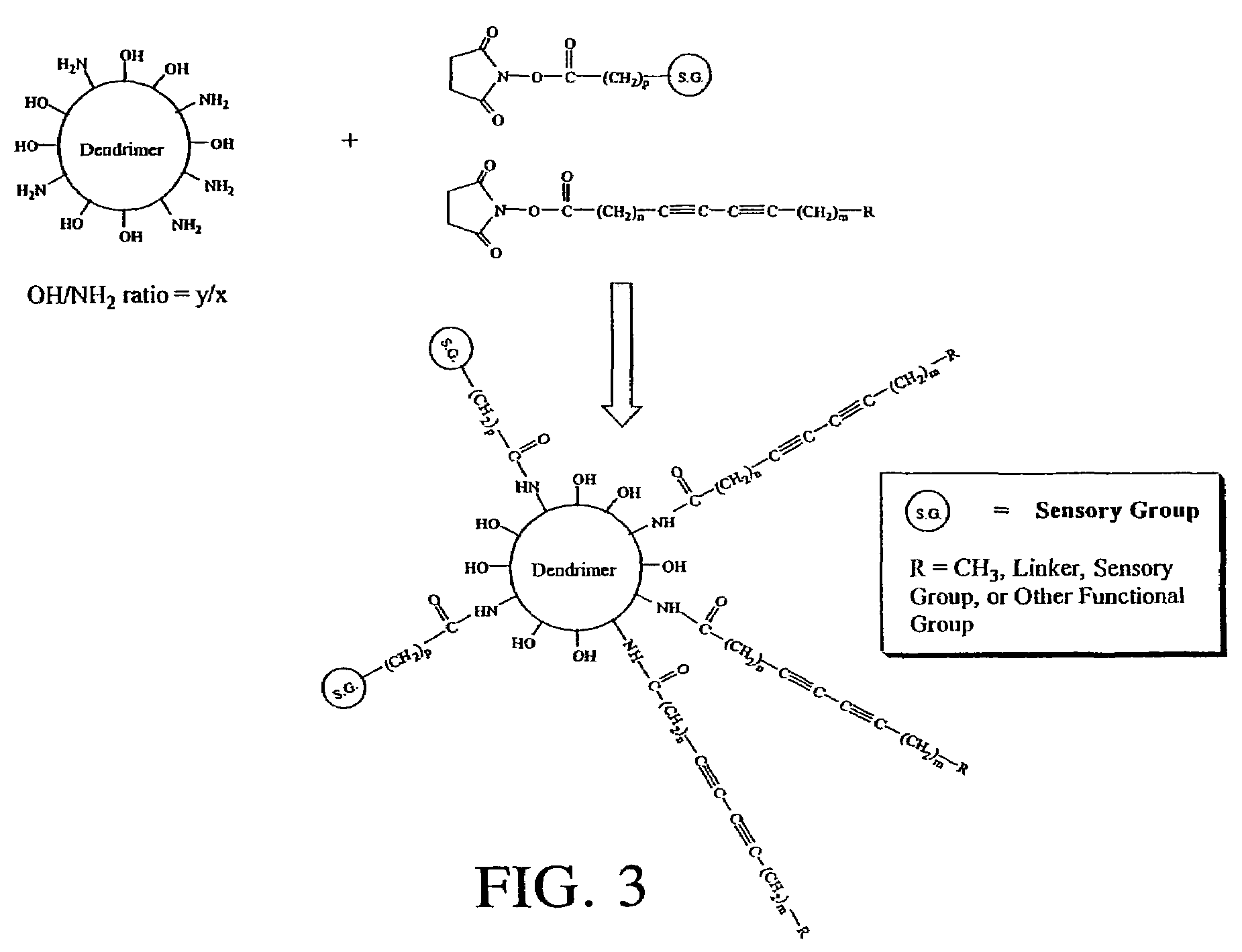
Solid-state colorimetric biosensors comprised of dendritic polymer networks
InactiveUS7141437B2Sugar derivativesMaterial analysis by observing effect on chemical indicatorCross-linkDendritic architecture
Owner:MICHIGAN MOLECULAR INST
Easily adhesive polyamide film
InactiveUS6352762B1High bonding strengthStrong adhesionLiquid surface applicatorsFilm/foil adhesivesWater basedPolyamide
An easily adhesive polyamide film has been created from unstretched or uniaxially stretched non-heated polyamide film coated with a water-base coating mixture, whose main constituents are (A) water polyurethane resin containing acetylene glycol in which each carbon atom immediately adjacent to the triple-bonded carbon atom is replaced with a hydroxyl group and a methyl group, and / or an ethylene oxide addition product of the acetylene glycol; (B) a water-soluble polyepoxy compound; and (C) particles with an average diameter between 0.001 and 1.0 mum, of which the solid-content weight ratio is 98-30 / 2-70 / 0.1-10, the coating amount after stretching is between 0.005 and 0.030 g / m2, and the film is stretched in at least one direction and then heated. This newly invented film possesses good blocking resistance and excellent adhesiveness with print ink, laminate, and other coating mixtures, and is especially suitable for boiling sterilization, retort sterilization, and packaging of liquids.
Owner:KOHJIN CO LTD
Photopolymerizable composition
InactiveUS20060024614A1High sensitivityRadiation applicationsSemiconductor/solid-state device manufacturingCompound (substance)Photoinitiator
A composition that is photopolymerizable upon absorption of light in the wavelength range from 300 to 450 nm, the composition comprising a binder, a polymerizable compound, a sensitizer and a photoinitiator, wherein the sensitizer is a fluorene compound that is conjugated via a double or triple bond with an aromatic or heteroaromatic group, and is characterized by a high sensitivity.
Owner:AGFA NV
Organometallic light-emitting material
InactiveUS7026480B2Increase brightnessImprove device efficiencyIndium organic compoundsDischarge tube luminescnet screensTriple bondCoordination complex
Disclosed herein are novel light-emitting materials of Formula I and II below. These new complexes are synthesized and found to be sufficiently stable to allow sublimation and vacuum deposition. These new emitters are electrophosphorescent and can be used in organic light-emitting devices (OLEDs) for device elements capable of emitting light of color ranging from orange to red with high-efficiency and high-brightness. wherein E=Group 16 elements (including sulphur); M=Group 10 metal (including platinum); R1-R14 are each independently selected from the group consisting of hydrogen; halogen; alkyl; substituted alkyl; aryl; substituted aryl, with substituents selected from the group consisting of halogen, lower alkyl and recognized donor and acceptor groups. R1 can also be selected from (C≡C)nR15, where (C≡C) represents a carbon-carbon triple bond (acetylide group), n is selected from 1 to 10, and R15 is selected from alkyl, aryl, substituted aryl, and tri(alkyl)silyl.
Owner:VERSITECH LTD
10,10-dialkyl prostanoic acid derivatives as agents for lowering intraocular pressure
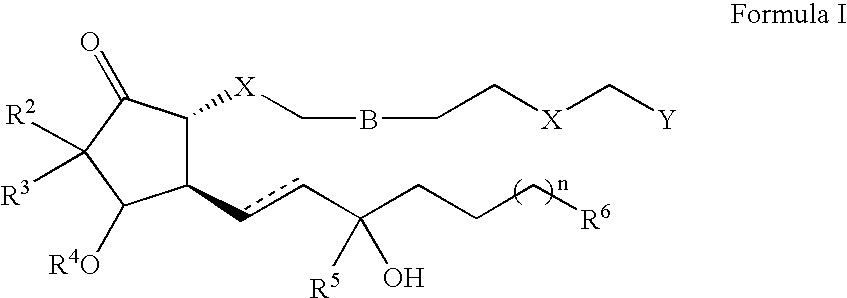
The present invention provides a method of treating ocular hypertension or glaucoma which comprises administering to an animal having ocular hypertension or glaucoma therapeutically effective amount of a compound represented by the general formula I; wherein the dashed line indicates the presence or absence of a bond, the hatched wedge indicates the α (down) configuration, and the solid triangle indicates the β (up) configuration;B is a single, double, or triple covalent bond;n is 0-6;X is CH2, S or O;Y is any pharmaceutically acceptable salt of CO2H, or CO2R, CONR2, NHCH2CH2OH, N(CH2CH2OH)2, CH2OR, P(O)(OR)2, CONRSO2R, SONR2, or R is H, C1-6 alkyl or C2-6 alkenyl;R2 and R3 are C1-6 linear alkyl which may be the same or different, and may be bonded to each other such that they form a ring incorporating the carbon to which they are commonly attached;R4 is hydrogen, R, C(═O)R, or any group that is easily removed under physiological conditions such that R4 is effectively hydrogen;R5 is hydrogen or R;R6 isiv) hydrogen;v) a linear or branched hydrocarbon containing between 1 and 8 carbon atoms, which may contain one or more double or triple bonds, or oxygen or halogen derivatives of said hydrocarbon, wherein 1-3 carbon or hydrogen atoms may be substituted by O or a halogen; orvi) aryloxy, heteroaryloxy, C3-8 cycloalkyloxy, C3-8 cycloalkyl, C6-10 aryl or C3-10 heteroaryl, wherein one or more carbons is substituted with N, O, or S; and which may contain one or more substituents selected from the group consisting of halogen, trihalomethyl, cyano, nitro, amino, hydroxy, C6-10 aryl, C3-10 heteroaryl, aryloxy, heteroaryloxy, C1-6 alkyl, OR, SR, and SO2R.Some of the compounds of the present invention and some of their methods of preparation are also novel an nonobvious.
Owner:ALLERGAN INC
Antibacterial fabric and preparing method thereof
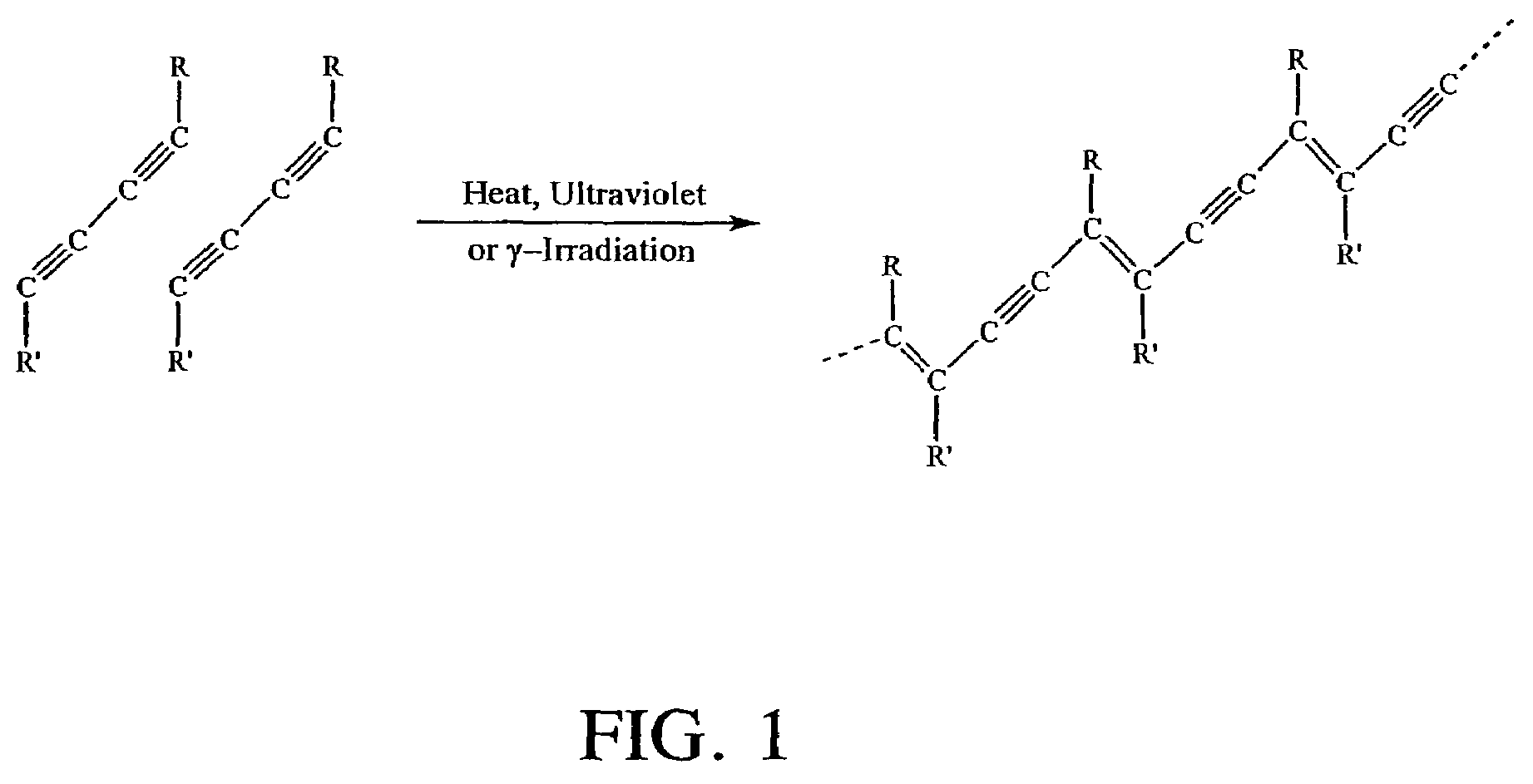
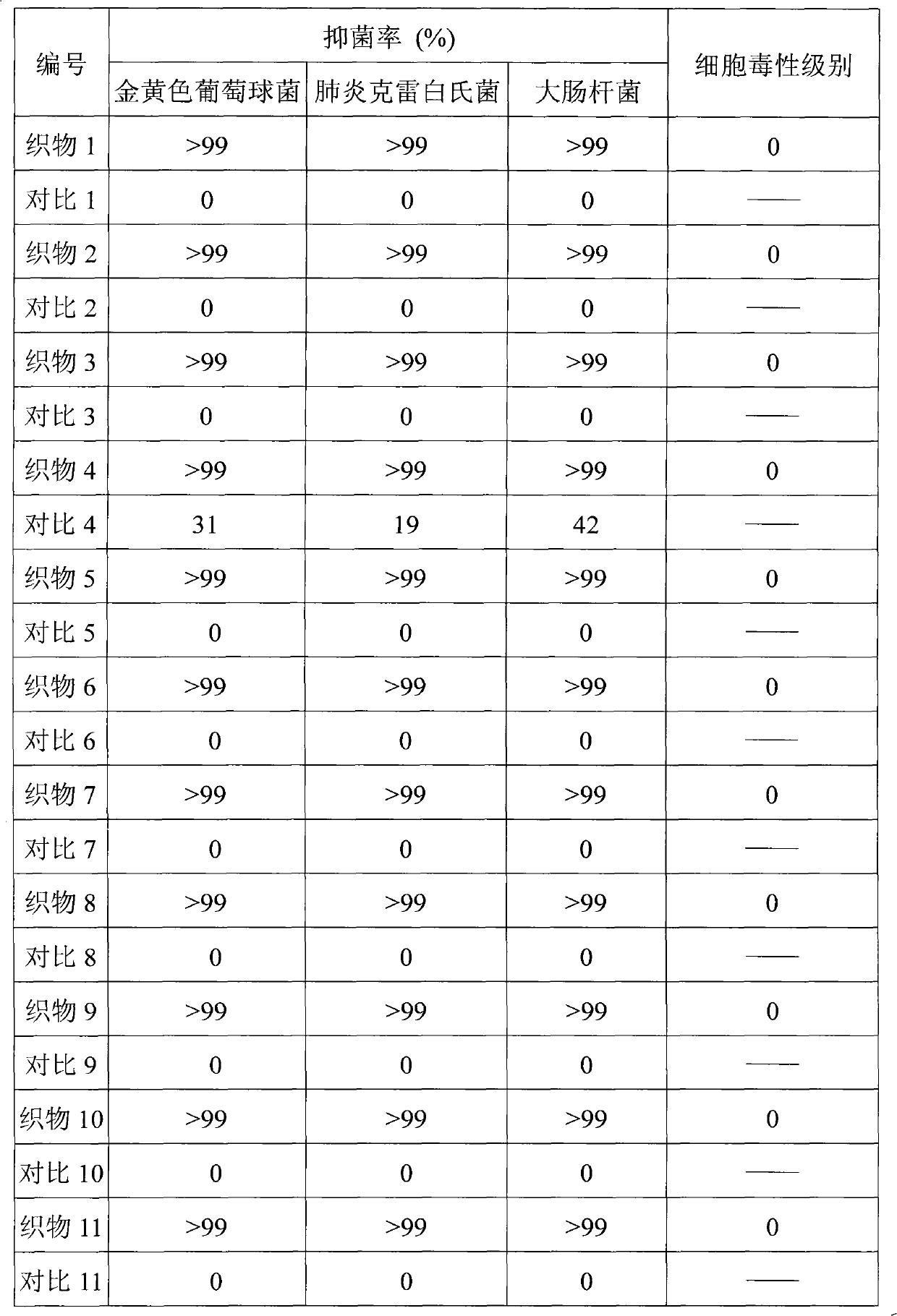
The invention relates to a type of antibacterial fabric and a preparing method thereof, with the preparing method comprising: adsorbing cross-linking agent to the surface of fabric containing C-H key to obtain the fabric adsorbing cross-linking agent; taking the fabric adsorbing cross-linking agent as filter cloth for filtering oxidation graphite alkene aqueous solution to obtain the fabric containing both the cross-linking agent and the oxidation graphite alkene; and employing radiation crosslinking method or heat crosslinking method to initiate the cross-linking agent upon the fabric containing both the cross-linking agent and the oxidation graphite alkene for crosslinking polymerization, thus to obtain antibacterial fabric, wherein the cross-linking agent contains more than two carbon-carbon double bonds and / or Carbon-carbon triple bonds. The method of the invention is easy to operate, consumes less amount of oxidation graphite alkene, uses less amount of time in post-treatment period, and is of low cost and suitable for large scale industrialized production. Moreover, the antibacterial fabric made in this way keeps great antibacterial performance even after repeated washing. With the cytotoxicity at Level 0 (the highest security level), the antibacterial fabric produces nearly no irritation to skin.
Owner:JINAN SHENGQUAN GROUP SHARE HLDG
Nonaqueous electrolyte solution and lithium secondary battery using same
ActiveUS20090053598A1Improve featuresReduce generationElectrolytic capacitorsOrganic electrolyte cellsCapacitanceAryl
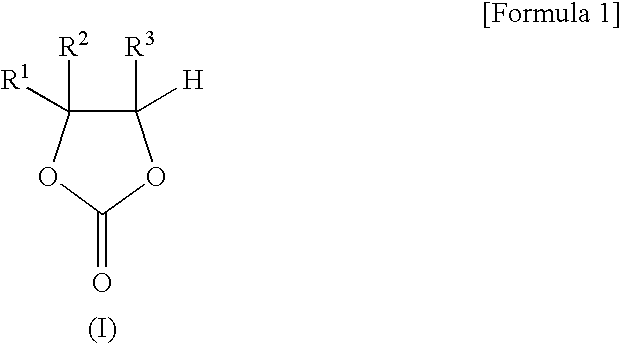
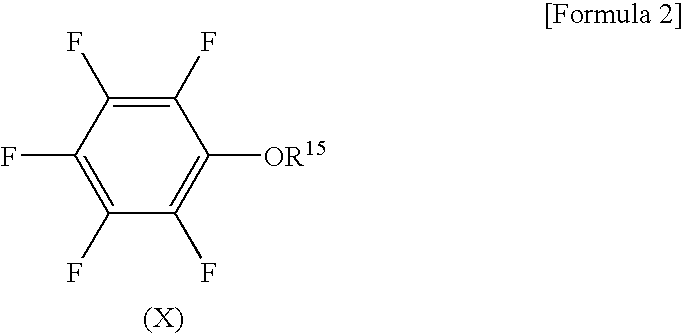
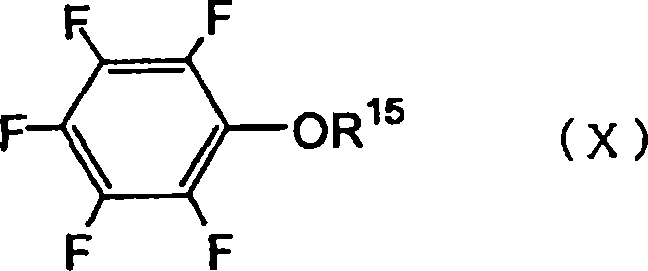
The present invention provides a nonaqueous electrolytic solution exhibiting excellent battery characteristics such as electrical capacity, cycle property and storage property and capable of maintaining the battery characteristics for a long tire, and a lithium secondary battery using the nonaqueous electrolytic solution.A nonaqueous electrolytic solution for a lithium secondary battery, in which an electrolyte salt is dissolved in a nonaqueous solvent, containing 0.1 to 10% by weight of an ethylene carbonate derivative represented by the general formula (I) shown below, and 0.01 to 10% by weight of (A) a triple bond-containing compound and / or (B) a pentafluorophenyloxy compound represented by the general formula (X) shown below:wherein R1 to R3 each independently represents a hydrogen atom, a halogen atom, an alkenyl group, an alkynyl group or an aryl group, provided that ethylene carbonate is excluded from the definition of the ethylene carbonate derivative,wherein R15 represents an alkylcarbonyl group, an alkoxycarbonyl group, an aryloxycarbonyl group or an alkanesulfonyl group.
Owner:MU IONIC SOLUTIONS CORP
Aliphatic-aromatic copolyester, preparation method and application thereof
ActiveCN101717493AAvoid problems such as affecting performanceEvenly dispersedAdhesivesCarboxylic acid halidesMonomer
The invention provides aliphatic-aromatic copolyester, a preparation method and application thereof. Polymerization monomers comprise a compound selected from aliphatic dibasic acid, and naphthenic base dibasic acid or the ester, the anhydride and the acyl halide thereof, a compound selected from aromatic dibasic acid or the ester, the anhydride and the acyl halide thereof, a compound simultaneously with two functional groups selected from an amino-group, a mercapto group or a hydroxy or a compound of a derivative of the amino-group, the mercapto group or the hydroxyl with an epoxy group and an azepine ring, a compound selected from unsaturated acid with at least one C-C, C-O, C-N or C-S double bond and C-C or C-N triple bond or the ester, the anhydride and the acyl halide thereof and a compound of unsaturated alcohol with at least one C-C double bond or a C-C triple bond or an epoxide thereof. The aliphatic-aromatic copolyester is prepared by carrying out esterification and polycondensation after mixing the polymerization monomers, polymerizing the double bonds and / or the triple bonds on the polymerization monomers under the action of an initiator and then carrying out a graftingand / or coupling reaction.
Owner:HANGZHOU XINFU TECH CO LTD
Oxo-azabicyclic compounds
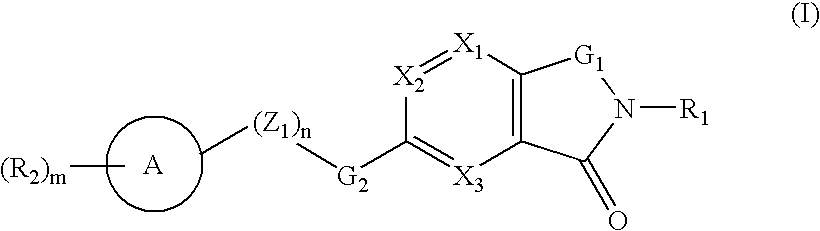
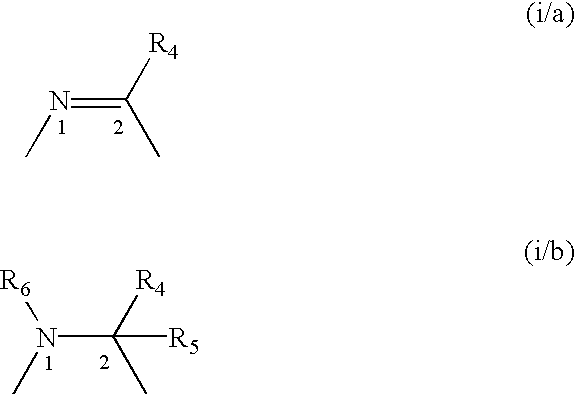
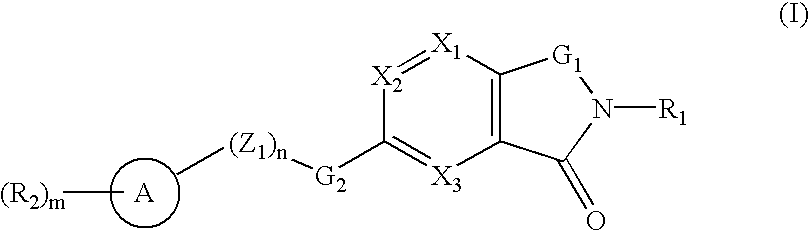
A compound selected from those of formula (I): wherein: X1, X2, and X3, represent N or -CR3 in which R3 is as described in the description, G1 represents a group selected from those of formulae (i / a) and (i / b): in which R4, R5, and R6 are as defined in the description, G2 represents a group selected from carbon-carbon triple bond, -CH=C=CH-, C=O, C=S, S(O)n1 in which n1 represents an integer from 0 to 2 inclusive, or a group of formula (i / c): in which Y1 represents O, S, -NH or -Nalkyl, and Y2 represents O, S, -NH or -Nalkyl, n is an integer from 0 to 6 inclusive, and m is an integer from 0 to 7 inclusive, Z1 represents -CR9R10, wherein R9 and R10 are as defined in the description, A represents a ring system, R1 represents a group selected from H, alkyl, alkenyl, alkynyl, optionally substituted and the group of formula (i / d): in which p, Z2, B, q and G3 are as defined in the description and optionally, its optical isomers, N-oxide, and addition salts thereof with a pharmaceutically-acceptable acid or base, and medicinal products containing the same are useful as specific inhibitors of type-13 matrix mettaloprotease.
Owner:WARNER-LAMBERT CO
Device comprising low outgassing photo or electron beam cured rubbery polymer material
Owner:CORNING INC
Aryl substituted 3,4-dihydroanthracene derivatives having retinoid antagonist or retinoid inverse agonist type biological activity
InactiveUS7166726B2Susceptibility to treatmentReduce adverse effectsSenses disorderNervous disorderArylSilylene
Disclosed herein are compounds of the formulawherein R1 is independently H or alkyl of 1 to 6 carbons;R2 is optional and is defined as lower alkyl of 1 to 6 carbons, F, Cl, Br, I, CF3, fluoro substituted alkyl of 1 to 6 carbons, OH, SH, alkoxy of 1 to 6 carbons, or alkylthio of 1 to 6 carbons;n is an integer of between 0 and 2;o is an integer between 0 and 3;R3 is hydrogen, lower alkyl of 1 to 6 carbons, F, Cl, Br or I;R4 is heteroaryl or (R5)p-heteroaryl where the heteroaryl group is 5-membered or 6-membered and has 1 to 3 heteroatoms selected from the group consisting of O, S, and N;p is an integer having the values of 0–5;R5 is F, Cl, Br, I, NO2, N(R8)2, N(R8)CORO8, N(R8)CON(R8)2, OH, OCOR8, OR8, CN, COOH, COOR8, C1-10 alkyl, fluoro substituted C1-10 alkyl, C2-10 alkenyl having 1 to 3 double bonds, C2-10 alkynyl having 1 to 3 triple bonds, or a (trialkyl)silyl or (trialkyl)silyloxy group where the alkyl groups independently have 1 to 6 carbons;A is (CH2)q where q is 0–5, C3-6 branched alkyl, C3-6 cycloalkyl, C2-6 alkenyl having 1 or 2 double bonds, or C2-6 alkynyl having 1 or 2 triple bonds;B is hydrogen, COOH or a pharmaceutically acceptable salt thereof, COORO8, CONR9R10, CH2OH, CH2OR11, CH2OCOR11, CHO, CH(OR12)2, CHOR13O, COR7, CR7(OR12)2, CR7OR13O, or Si(C1-6alkyl)3;R7 is C1-5 alkyl, C3-5 cycloalkyl, or C2-5 alkenyl;R8 is C1-10 alkyl, C1-10 (trimethylsilyl)alkyl, or C5-10 cycloalkyl;R9 and R10 are independently hydrogen, C1-10 alkyl, C5-10 cycloalkyl, phenyl or R12-phenyl;R11 is C1-6 alkyl, phenyl, or R12-phenyl;R12 is C1-6 alkyl; andR13 is divalent alkyl radical of 2–5 carbons.
Owner:ALLERGAN INC
10,10-dialkyl prostanoic acid derivatives as agents for lowering intraocular pressure
The present invention provides a method of treating ocular hypertension or glaucoma which comprises administering to an animal having ocular hypertension or glaucoma therapeutically effective amount of a compound represented by the general formula I; wherein the dashed line indicates the presence or absence of a bond, the hatched wedge indicates the alpha (down) configuration, and the solid triangle indicates the beta (up) configuration; B is a single, double, or triple covalent bond; n is 0-6; X is CH2, S or O; Y is any pharmaceutically acceptable salt of CO2H, or CO2R, CONR2, CONHCH2CH2OH, CON(CH2CH2OH)2,CH2OR, P(O)(OR)2, CONRSO2R, SONR2, or R is H, C1-6 alkyl or C2-6 alkenyl; R<2 >and R<3 >are C1-6 linear alkyl which may be the same or different, and may be bonded to each other such that they form a ring incorporating the carbon to which they are commonly attached; R<4>is hydrogen, R, C(=O)R, or any group that is easily removed under physiological conditions such that R<4 >is effectively hydrogen; R<5 >is hydrogen or R; R<6 >is i) hydrogen; ii) a linear or branched hydrocarbon containing between 1 and 8 carbon atoms, which may contain one or more double or triple bonds, or oxygen or halogen derivatives of said hydrocarbon, wherein 1-3 carbon or hydrogen atoms may be substituted by O or a halogen; or iii) aryloxy, heteroaryloxy, C3-8 cycloalkyloxy, C3-8 cycloalkyl, C6-10 aryl or C3-10 heteroaryl, wherein one or more carbons is substituted with N, O, or S; and which may contain one or more substituents selected from the group consisting of halogen, trihalomethyl, cyano, nitro, amino, hydroxy, C6-10 aryl, C3-10 heteroaryl, aryloxy, heteroaryloxy, C1-6 alkyl, OR, SR, and SO2R. Some of the compounds of the present invention and some of their methods of preparation are also novel an nonobvious.
Owner:ALLERGAN INC
Carbon black
ActiveUS7300964B2Improve dynamic rigidityReduce hysteresisOrganic chemistryOther chemical processesOrganic groupOrganic compound
Carbon black having organic groups, the organic group containing a thiocyanate group. Also described is a process for the production of the carbon black, wherein carbon black is reacted with organic compounds containing a C—C double or triple bond, which is not part of an aromatic system, whose C—C double or triple bond is activated by at least one substituent, and the organic compound contains at least one thiocyanate group. The carbon black according to the invention can be used in rubber compounds.
Owner:UBS AG
Oxo-azabicyclic compounds
A compound selected from those of formula (I): wherein: X1, X2, and X3, represent N or -CR3 in which R3 is as described in the description, G1 represents a group selected from those of formulae (i / a) and (i / b): in which R4, R5, and R6 are as defined in the description, G2 represents a group selected from carbon-carbon triple bond, -CH=C=CH-, C=O, C=S, S(O)n1 in which n1 represents an integer from 0 to 2 inclusive, or a group of formula (i / c): in which Y1 represents O, S, -NH or -Nalkyl, and Y2 represents O, S, -NH or -Nalkyl, n is an integer from 0 to 6 inclusive, and m is an integer from 0 to 7 inclusive, Z1 represents -CR9R10 wherein R9 and R10 are as defined in the description, A represents a ring system, R1 represents a group selected from H, alkyl, alkenyl, alkynyl, optionally substituted and the group of formula (i / d): in which p, Z2, B, q and G3 are as defined in the description and optionally, its optical isomers, N-oxide, and addition salts thereof with a pharmaceutically-acceptable acid or base, and medicinal products containing the same are useful as specific inhibitors of type-13 matrix mettaloprotease.
Owner:WARNER-LAMBERT CO
Aqueous dispersion for chemical mechanical polishing, chemical mechanical polishing method, kit for chemical mechanical polishing, and kit for preparing aqueous dispersion for chemical mechanical polishing
InactiveUS20090124172A1Efficient polishingSufficiently planarized and accurately finishedOther chemical processesSemiconductor/solid-state device manufacturingOrganic acidMetallurgy
A chemical mechanical polishing aqueous dispersion comprises (A) abrasive grains, (B) at least one of quinolinecarboxylic acid and pyridinecarboxylic acid, (C) an organic acid other than quinolinecarboxylic acid and pyridinecarboxylic acid, (D) an oxidizing agent, and (E) a nonionic surfactant having a triple bond, the mass ratio (WB / WC) of the amount (WB) of the component (B) to the amount (WC) of the component (C) being 0.01 or more and less than 2, and the component (E) being shown by the following general formula (1),wherein m and n individually represent integers equal to or larger than one, provided that m+n≦50 is satisfied.
Owner:JSR CORPORATIOON +1
Hydrogenation catalyst steeping fluid composition and preparation method of hydrogenation catalyst
ActiveCN101462078AImprove hydrogenation activityHigh cracking activityCatalyst activation/preparationHydrocarbon oil crackingReaction temperatureFluid composition
The invention provides a hydrogenation catalyst soaking solution composition, which comprises a precursor of a hydrogenation active ingredient, a soaking additive and water, wherein the soaking additive has similar pKa value with that of the precursor of the hydrogenation active ingredient and comprises carbon-carbon double bond and / or carbon-carbon triple bond substances in molecular structure. The invention also provides a method for preparing a hydrogenation catalyst, which comprises: using the soaking solution to soak a catalyst carrier, and drying and roasting the catalyst carrier, wherein the soaking solution is the hydrogenation catalyst soaking solution composition. The hydrogenation catalyst prepared from the hydrogenation catalyst soaking solution composition has higher hydrogenation activity and higher cracking activity at the same reaction temperature compared with a hydrogenation catalyst prepared by the prior art.
Owner:CHINA PETROLEUM & CHEM CORP +1
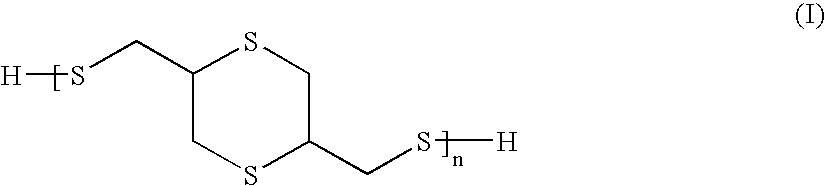
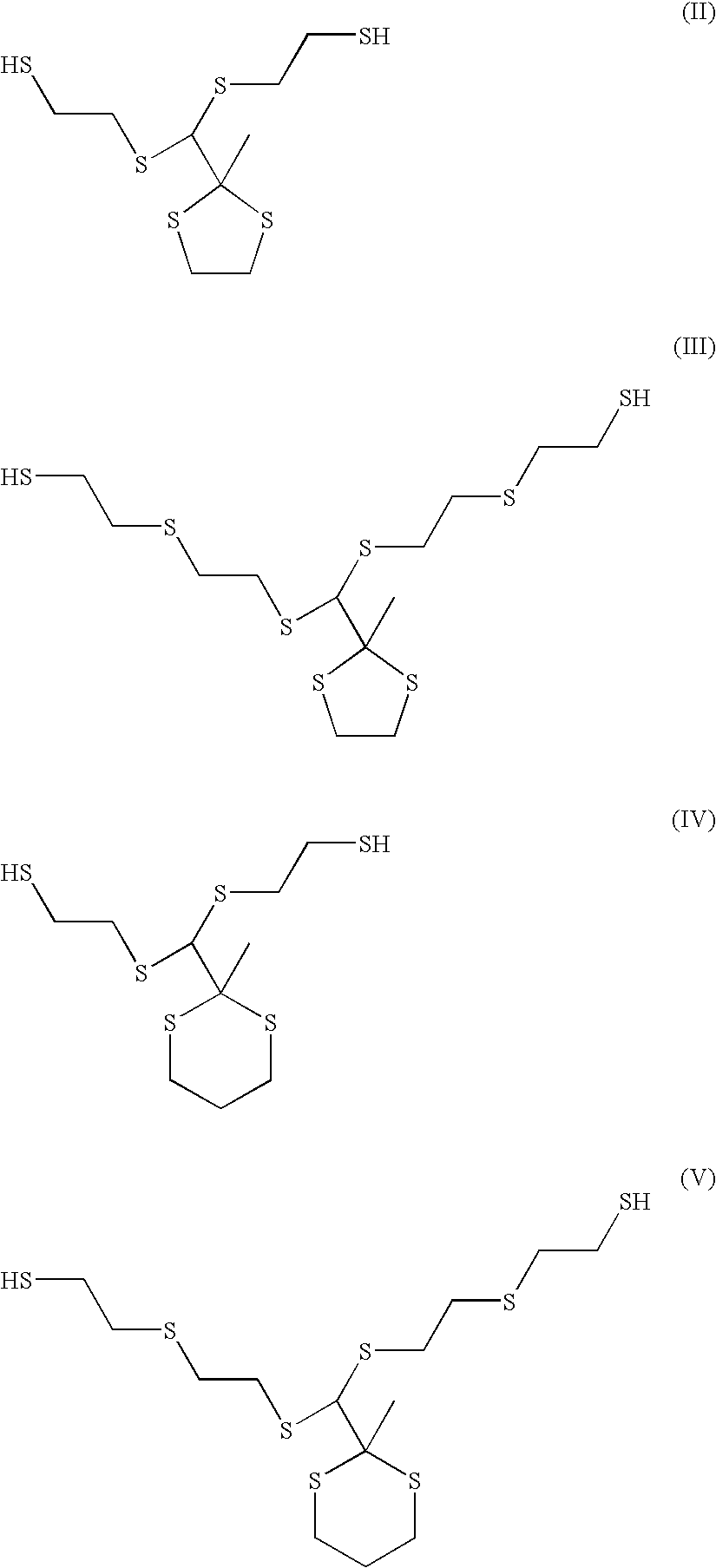
Compositions and articles prepared from thioether functional oligomeric polythiols
Provided is a composition including a reaction product of: (A) a reactive compound that includes a material having functional groups that are reactive with active hydrogens; (B) a thioether-functional, oligomeric polythiol prepared by reacting together: (1) a compound having at least two thiol functional groups; (2) a compound having triple bond functionality; and optionally (3) a compound having at least two double bonds; and, optionally, (C) a compound different from (B) containing active hydrogens. Coating compositions, articles of manufacture and related processes also are provided.
Owner:PPG IND OHIO INC
Photo-alignment film and liquid crystal display element
InactiveUS20070232780A1Good in alignment stabilityHigh voltage retentionLiquid crystal compositionsThin material handlingLiquid-crystal displayPolyamide
[Object] To obtain an alignment film having excellent alignment stability of a liquid crystal and a high voltage holding ratio by application of linearly polarized light to a polyamic acid having a specific structure and then imidization under heat. [Solving Means] A photo-alignment film is obtained by: applying a polyamic acid solution on a substrate, where the polyamic acid contains, in its main chain, at least a group having unsaturated groups having 1 to 3 carbon-carbon double bonds or 1 to 4 triple bonds; vaporizing a solvent from a film formed; applying linearly polarized light to the film after the vaporization of the solvent; and then heating the film to imidize the polyamic acid.
Owner:JNC PETROCHEM CORP +1
Methods for improving integration performance of low stress CDO films
Methods of preparing a carbon doped oxide (CDO) layer with a low dielectric constant (<3) and low residual stress without sacrificing important integration properties such as dry etch rate, film stability during wet cleaning, electrical leakage current, and extinction coefficient are provided. The methods involve, for instance, providing a substrate to a deposition chamber and exposing it to a chemical precursor having molecules with at least one carbon-carbon triple bond, followed by igniting and maintaining a plasma in a deposition chamber using radio frequency power having high and low frequency components or one frequency component only, and depositing the carbon doped oxide film under conditions in which the resulting dielectric layer has a compressive stress or a tensile stress of between about −20 to 30 MPa and a dielectric constant of between about 2.5-3.0, a C≡C to SiO bond ratio of between about 0.05% to 5%, a SiC to SiO bond ratio of between about 2% to 10%, and a refractive index (RI) of 1.39-1.52 measured at 633 nm.
Owner:NOVELLUS SYSTEMS
Nonaqueous electrolyte solution and lithium secondary battery using same
ActiveCN101107745AIncrease capacityExcellent cycle characteristicsSecondary cellsActive material electrodesArylHalogen
Disclosed is a nonaqueous electrolyte solution which is excellent in battery characteristics such as battery capacity, cycle characteristics and storage characteristics and is capable of maintaining adequate battery performance for a long time. Also disclosed is a lithium secondary battery using such a nonaqueous electrolyte solution. Specifically disclosed is a nonaqueous electrolyte solution for lithium secondary batteries wherein an electrolyte salt is dissolved in a nonaqueous solvent. This nonaqueous electrolyte solution contains 0.1-10% by weight of an ethylene carbonate derivative represented by the general formula (I) below, and 0.01-10% by weight of a triple bond-containing compound (A) and / or a pentafluorophenyloxy compound (B) represented by the general formula (X) below. Also specifically disclosed is a lithium secondary battery using such a nonaqueous electrolyte solution. [Chemical formula 1] (I) (In the formula (I), R<1>-R<3> respectively represent a hydrogen atom, a halogen atom, an alkenyl group, an alkynyl group or an aryl group. In this connection, ethylene carbonates are excluded.) [Chemical formula 2] (X) (In the formula (X), R<15> represents an alkylcarbonyl group, an alkoxycarbonyl group, an aryloxycarbonyl group or an alkanesulfonyl group.
Owner:MU IONIC SOLUTIONS CORP
Photosensitive resin composition, electronic component using the same, and display using same
ActiveUS20060159839A1Improve crack resistanceGood heat shrinkabilityPhotosensitive materialsRadiation applicationsDisplay deviceElectronic component
This invention relates to a negative-working photosensitive resin composition that can be developed in an alkaline developer. This photosensitive resin composition comprises: (a) a polyimide having at least one group selected from the group consisting of a carboxyl group, a phenolic hydroxyl group, a sulfonic acid group, and a thiol group at the terminus of the polymer main chain; (b) a compound having a polymerizable functional group comprising unsaturated double and / or triple bonds; and (c) a photopolymerization initiator.
Owner:TORAY IND INC
Process for hydrogenating cuts containing hydrocarbons, in particular unsaturated molecules containing at least two double bonds or at least one triple bond
Hydrogenation of a liquid cut containing hydrocarbons, in particular unsaturated molecules containing at least two double bonds or at least one triple bond, is described wherein the unsaturated molecules are at least partially hydrogenated to less unsaturated molecules containing at least one double bond, in at least one reactor comprising at least two distinct beds of at least one hydrogenation catalyst, and wherein a gas phase containing hydrogen is introduced, a portion thereof being mixed with said cut upstream of the first catalyst bed and a portion thereof being introduced upstream of the subsequent beds contained in said reactor.
Owner:INST FR DU PETROLE
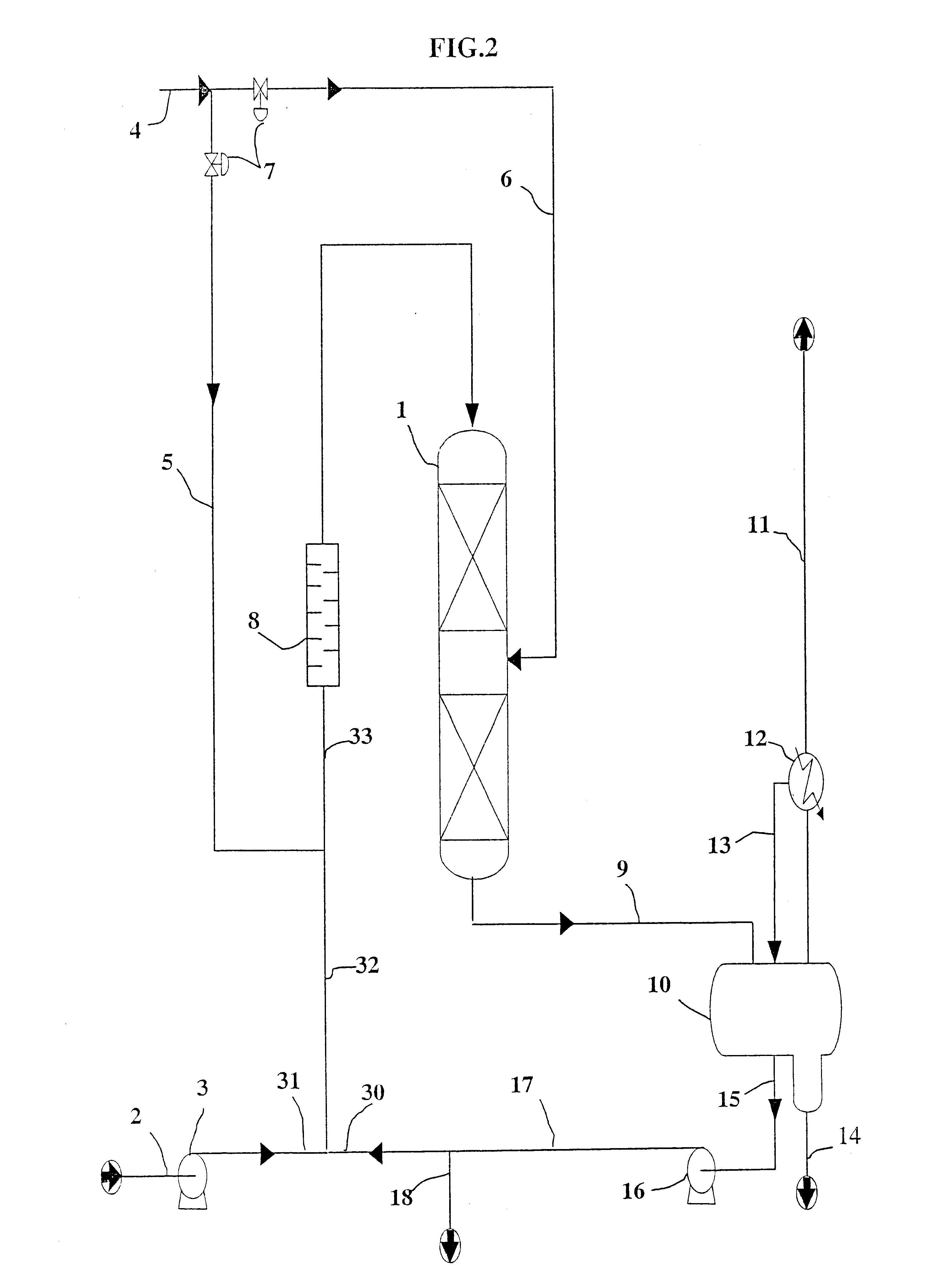
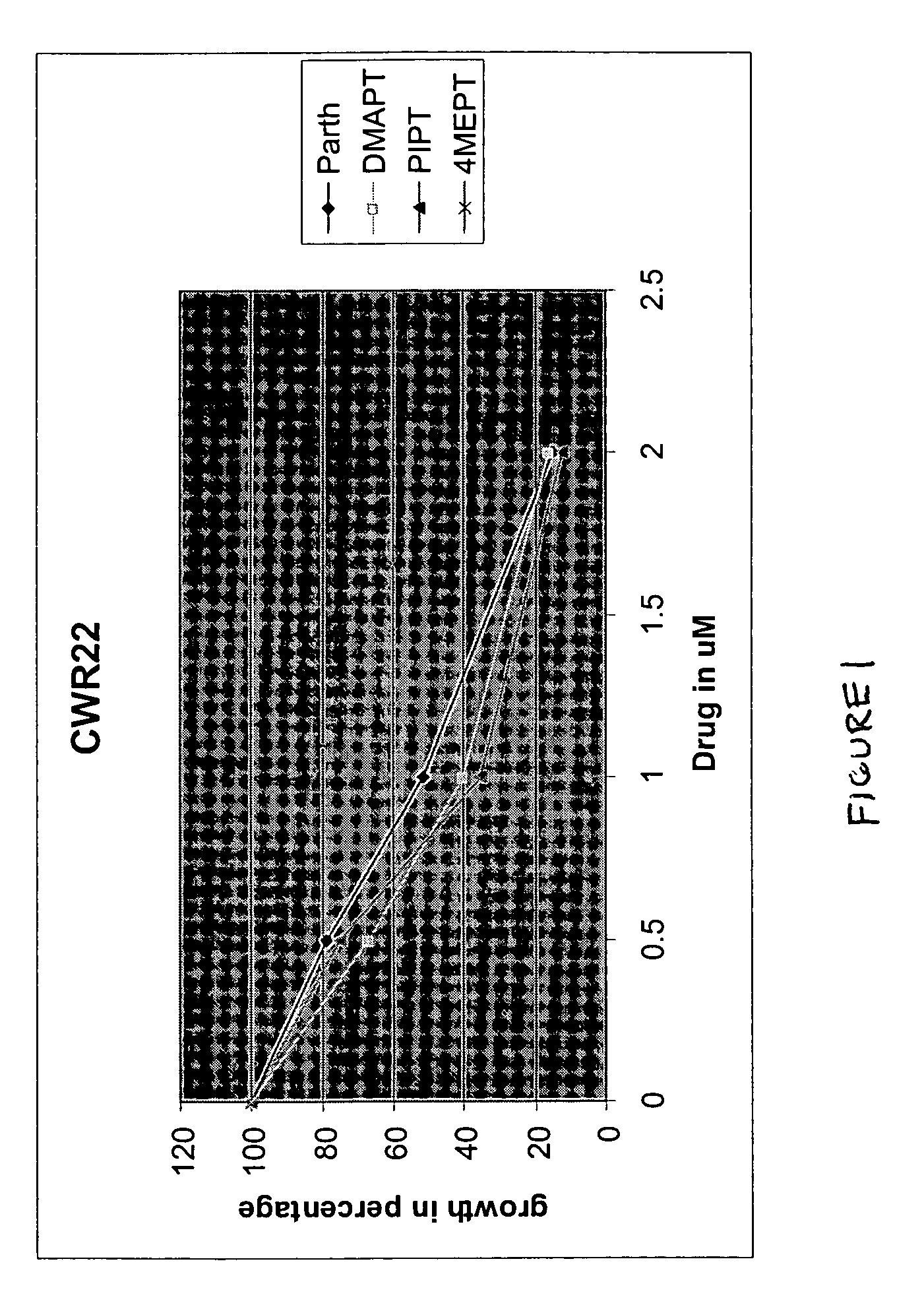
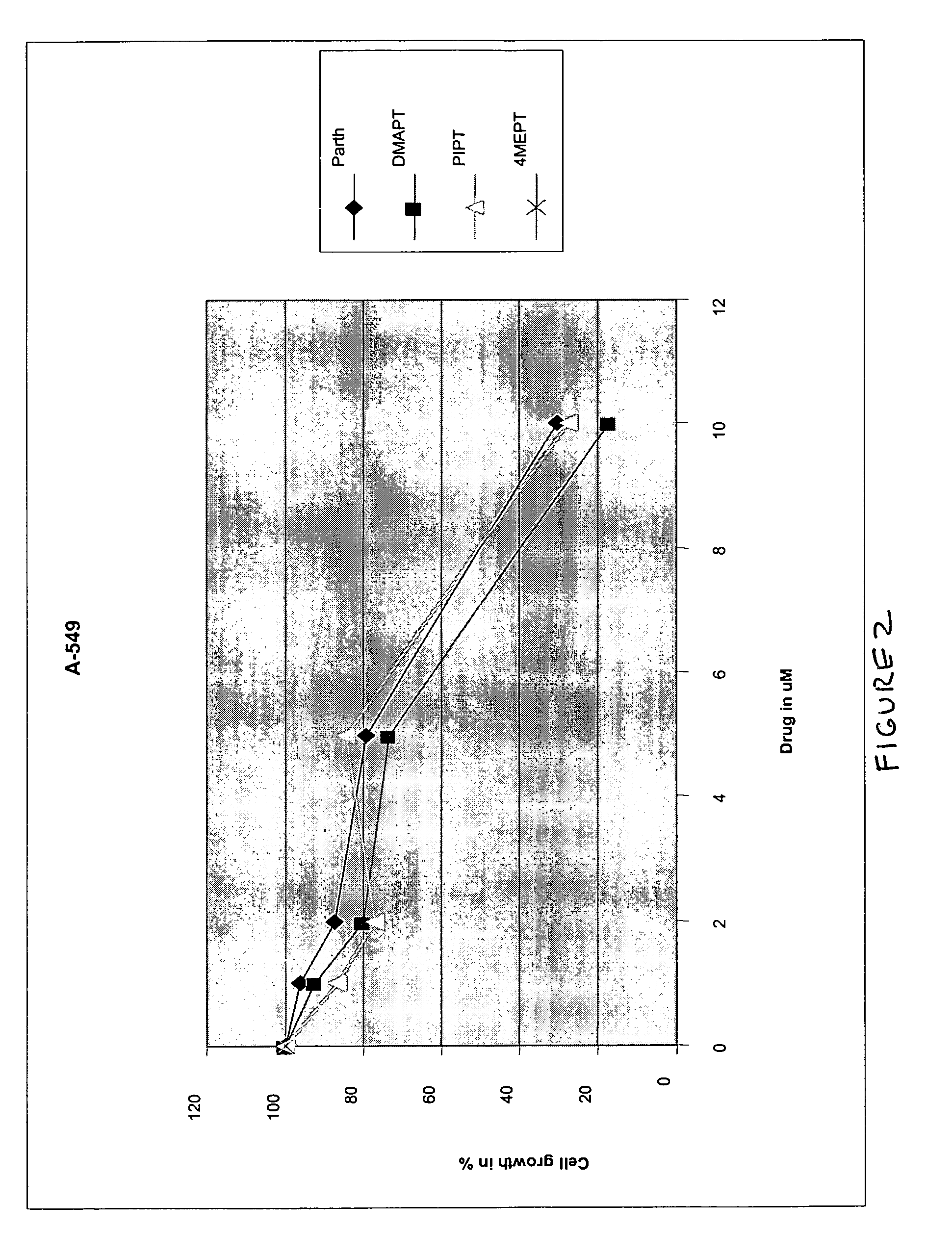
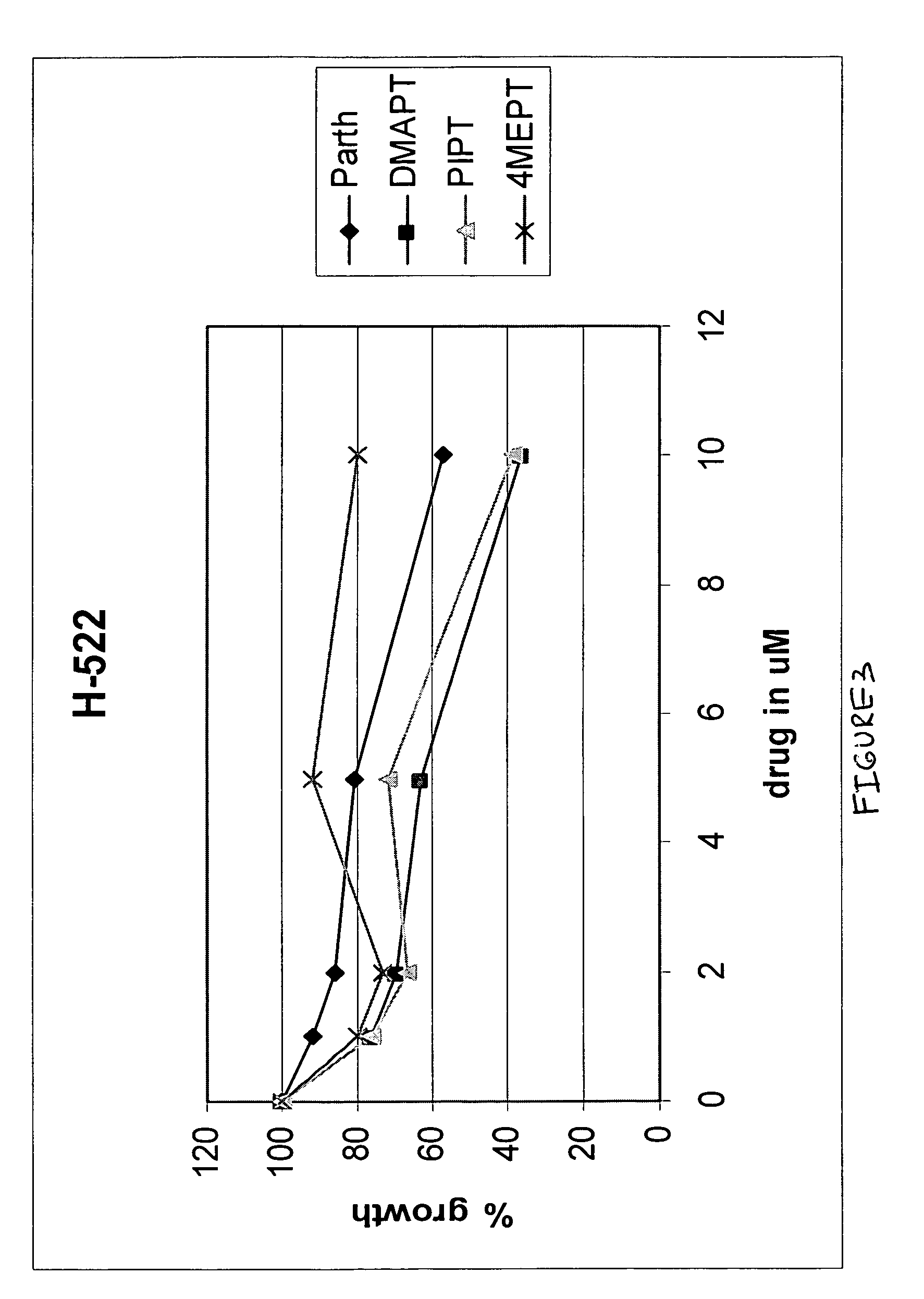
Use of parthenolide derivatives as antileukemic and cytotoxic agents
The present invention provides compounds of the formula (I)wherein:X1, X2 and X3 are heteroatoms;R4, R5, R6, R7, R8, R9 and R10 are independently selected from H, halo, —OH, —NO2, —CN and optionally substituted aliphatic, cycloalkyl, heterocycloalkyl, aryl or heteroaryl; andZ is optionally substituted C1-8 straight-chained or branched aliphatic, optionally containing 1 or more double or triple bonds, wherein one or more carbons are optionally replaced by R* wherein R* is optionally substituted cycloalkyl, heterocycloalkyl, aryl or heteroaryl; an amino acid residue, H, —CN, —C(O)—, —C(O)C(O)—, —C(O)NR1—, —C(O)NR1NR2—, —C(O)O—, —OC(O)—, —NR1CO2—, —O—, —NR1C(O)NR2—, —OC(O)NR1—, —NR1NR2—, —NR1C(O)—, —S—, —SO—, —SO2—, —NR1—, —SO2NR1—, —NR1R2, or —NR1SO2—, wherein R1 and R2 are independently selected from H and optionally substituted aliphatic, cycloalkyl, heterocycloalkyl, aryl or heteroaryl; or where R* is NR1R2, R1 and R2 optionally together with the nitrogen atom form an optionally substituted 5-12 membered ring, said ring optionally comprising 1 or more heteroatoms or a group selected from —CO—, —SO—, —SO2— and —PO—; ora pharmaceutically acceptable salt, ester or prodrug thereof.
Owner:KENTUCKY UNIVERISTY OF
Non-aqueous electrolyte of lithium ion battery and lithium ion battery
InactiveCN105161763AImprove high temperature performanceLower impedanceSecondary cellsPhosphateLithium-ion battery
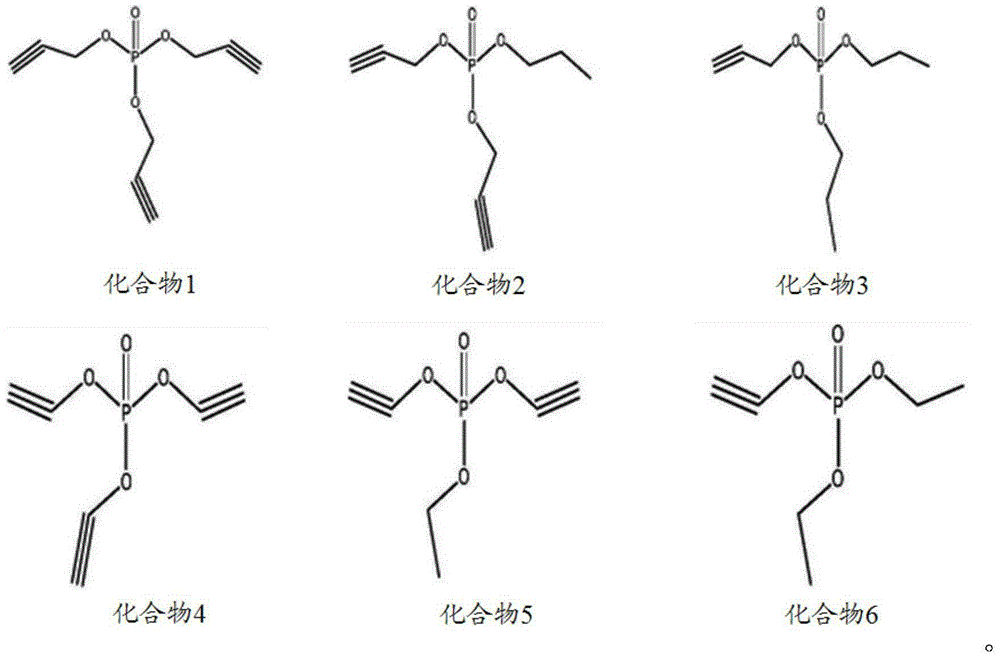
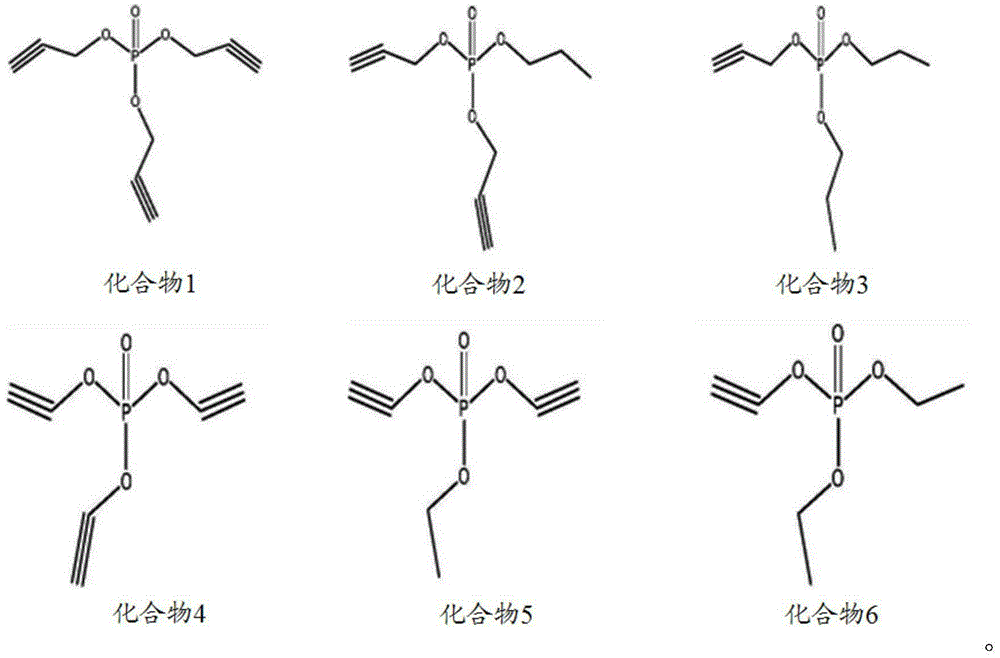
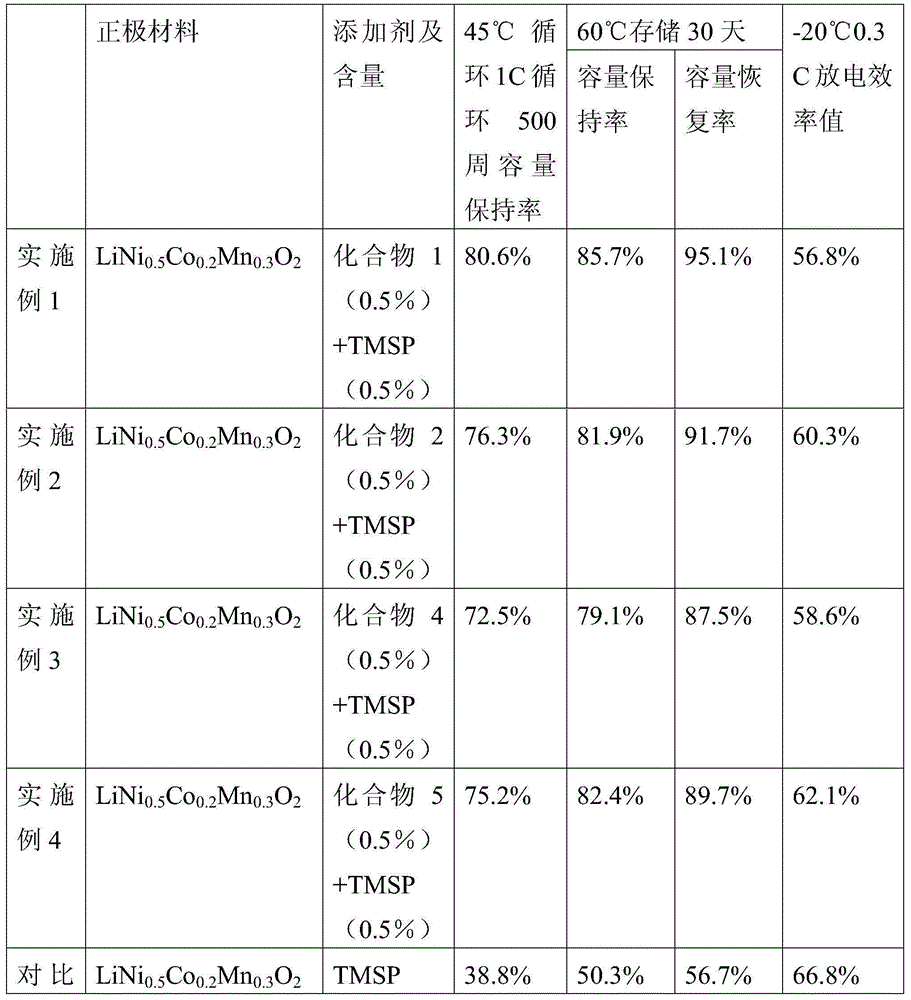
The invention discloses a non-aqueous electrolyte of a lithium ion battery and the lithium ion battery. The electrolyte comprises a non-aqueous organic solvent, a lithium salt and an additive, wherein the additive comprises substances of (A) and (B): (A) is shown in the description, wherein R1, R2 and R3 are respectively and independently selected from alkyl with carbon atoms of 1to 4, and at least one of the R1, the R2 and the R3 is unsaturated alkyl containing triple bonds; and (B) tris(trimethylsilyl) phosphate. By the non-aqueous electrolyte of the lithium ion battery, disclosed by the invention, the lithium ion battery acquires low impedance, and favorable low-temperature performance and high-temperature performance.
Owner:SHENZHEN CAPCHEM TECH CO LTD
Method for producing semiconductor including forming a layer containing at least silicon carbide and forming a second layer containing at least silicon oxygen carbide
InactiveUS6838393B2Semiconductor/solid-state device manufacturingChemical vapor deposition coatingSilicon oxygenOxygen
Methods are provided for depositing a silicon carbide layer having significantly reduced current leakage. The silicon carbide layer may be a barrier layer or part of a barrier bilayer that also includes a barrier layer. Methods for depositing oxygen-doped silicon carbide barrier layers are also provided. The silicon carbide layer may be deposited by reacting a gas mixture comprising an organosilicon compound, an aliphatic hydrocarbon comprising a carbon-carbon double bond or a carbon-carbon triple bond, and optionally, helium in a plasma. Alternatively, the silicon carbide layer may be deposited by reacting a gas mixture comprising hydrogen or argon and an organosilicon compound in a plasma.
Owner:APPLIED MATERIALS INC
Benzodifuran conjugated polymer material and preparation method and application thereof
ActiveCN102408547ASolid-state devicesSemiconductor/solid-state device manufacturingOrganic solar cellField-effect transistor
The invention discloses a benzodifuran conjugated polymer material and a preparation method and application thereof. The structural formula of the polymer is shown by a formula I, wherein A1, A2, R1 and R2 separately represent hydrogen, alkyl group with 1-30 carbon atoms, alkoxyl group with 1-30 carbon atoms, cyano group, nitro group, carbonyl group, ester group, aryl group, aralkyl group, halogen, haloalkyl, heteroalkyl, alkenyl, and aryl or conjugation unit substituted by a substituent group containing single bond, double bond, triple bond or the combination thereof; and Ar is selected fromthe groups substituted or unsubstituted: vinylidene, ethynylene, monocycle arylene, bicyclo-arylene, arylene with three or more rings, monocyclic heteroarylene, bicyclo-heteroarylene, heteroarylene with three or more rings and 1-6 of the groups condensed or connected. The polymer material disclosed by the invention can be applied to the photoelectric field such as organic solar cells and high-shift field effect transistors. The formula I is shown in the specification.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Aqueous dispersion for chemical mechanical polishing, chemical mechanical polishing method, kit for chemical mechanical polishing, and kit for preparing aqueous dispersion for chemical mechanical polishing
InactiveUS20110250756A1Efficient polishingSufficiently planarizedOther chemical processesSemiconductor/solid-state device manufacturingOrganic acidMetallurgy
A chemical mechanical polishing aqueous dispersion comprises (A) abrasive grains, (B) at least one of quinolinecarboxylic acid and pyridinecarboxylic acid, (C) an organic acid other than quinolinecarboxylic acid and pyridinecarboxylic acid, (D) an oxidizing agent, and (E) a nonionic surfactant having a triple bond, the mass ratio (WB / WC) of the amount (WB) of the component (B) to the amount (WC) of the component (C) being 0.01 or more and less than 2, and the component (E) being shown by the following general formula (1),wherein m and n individually represent integers equal to or larger than one, provided that m+n≦50 is satisfied.
Owner:KK TOSHIBA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com