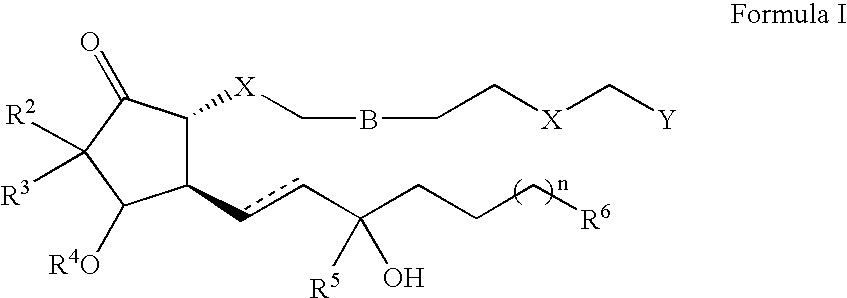
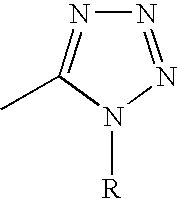
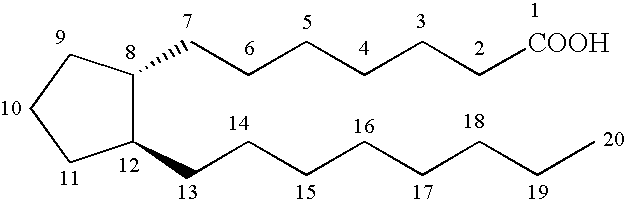
10,10-dialkyl prostanoic acid derivatives as agents for lowering intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0109] The methods of preparing compounds of this invention are further illustrated by the following non-limiting Examples, which are summarized in the reaction schemes of FIGS. 1-7 wherein the compounds are identified by the same designator in both the Examples and the Figures.
[0110] 2-Alkyl-cyclopentane-1,3-dione (1a). A mixture of 1,3-cyclopentanedione (89.4 mmol, Aldrich), I--R.sup.2 (96.4 mmol, Aldrich), and KOH (5.097 g, 90.8 mmol) in H.sub.2O (25 mL) / dioxane (75 mL) is heated at reflux. After 5 h, a solution of KOH (2 g) and I--R.sup.2 (2 mmol) in H.sub.2O (5 mL) / dioxane (15 mL) is added and after another 3 h at reflux the solution is allowed to stir at room temperature overnight. In the morning, the reaction is continued by addition of a solution of KOH (2 g) and I--R.sup.2 (2.4 mmol) in H.sub.2O (5 mL) / dioxane (15 mL) and heating at reflux. After 4 h, the mixture is allowed to cool to room temperature and is extracted with ether (1.times.100 mL, 3.times.75 mL). The combined...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com