Spirobi(dihydrobenzosilole) diphenol compound, and synthesis method and application thereof
A technology of spirobisdihydrobenzothiazole diphenols and compounds, which is applied in the field of spirobisdihydrobenzothiazole diphenols and can solve the problem of single ligands and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
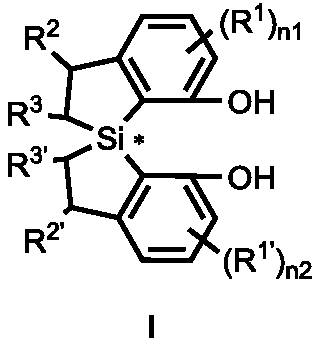
[0392] Example 1: Synthesis of chiral diphenol compounds with spirobisdihydrobenzosilole skeleton
[0393]
[0394] step 1)
[0395] In a nitrogen atmosphere, methyltriphenylphosphine bromide (15.25g, 42.7mmol) and potassium tert-butoxide (6.0g, 53,4mmol) and 50mL tetrahydrofuran were mixed with a 200mL dry Schlenk bottle, and stirred at room temperature for 0.5h , the tetrahydrofuran solution dissolved in 1-(2-bromo-3-methoxyphenyl)ethanone (8.12g, 35.6mmol) was added dropwise to the previous step system, TLC detected that the reaction was complete, and quenched by dropping saturated ammonium chloride aqueous solution reaction, extracted three times with ethyl acetate, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, allowed to stand, and the desiccant was removed by suction filtration, and the filtrate was purified by column chromatography to obtain 2-bromo-1 -Methoxy-3-(prop-1-en-2-yl)benzene (7.07 g, yield: 88%).
[0396] step (2...
Embodiment 2
[0408] Embodiment 2: I-2
[0409]
[0410] Use (2S,4S)-2,4-bis(diphenylphosphonium)pentane to replace (2R,4R)-2,4-bis(diphenylphosphonium)pentane, with reference to the reaction conditions in Example 1 and The method of operation is prepared.
[0411] White solid, 96% yield, [a] D 24 =-4.23 (c=0.90, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.28(t, J=7.8Hz, 2H), 6.95(d, J=7.6Hz, 2H), 6.56(d, J=7.9Hz, 2H), 4.51(s, 2H), 3.60–3.42( m,2H),1.75(dd,J=15.1,8.1Hz,2H),1.38(d,J=7.0Hz,6H),0.94(dd,J=15.1,4.7Hz,2H); 13 C NMR (100MHz, CDCl 3 )δ161.58, 159.71, 132.73, 120.39, 117.88, 112.02, 38.65, 25.46, 20.44; HRMS (ESI-TOF) m / z Calcd for C 18 h 21 o 2 Si[M+H] + :297.1305,found:297.1304.
[0412] Use corresponding raw material, with reference to reaction condition and operation method in embodiment 1 or 2, prepare following compound:
Embodiment 3
[0413] Embodiment 3: I-3
[0414]
[0415] White solid, 92% yield, [a] D 25 =+3.5 (c=1.07, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.29–7.19(m,2H),6.91(d,J=7.5Hz,2H),6.53(d,J=7.9Hz,2H),4.59(s,2H),3.38–3.06(m,4H ),1.51–1.35(m,2H),1.31–1.13(m,2H); 13 C NMR (100MHz, CDCl 3)δ160.03, 156.69, 132.60, 121.38, 118.75, 111.83, 31.67, 9.40; HRMS (ESI-TOF) m / z Calcd for C 16 h 17 o 2 NaSi[M+Na] + :291.0812,found:291.0815.
[0416] Single crystal culture conditions: Dissolve 10 mg of the compound in 0.5 mL of dichloromethane in an 8 mL vial, add 4 mL of n-hexane, cover the bottle with a hole, and let it stand for 0.5-1 day. The crystal was subjected to single crystal diffraction test.
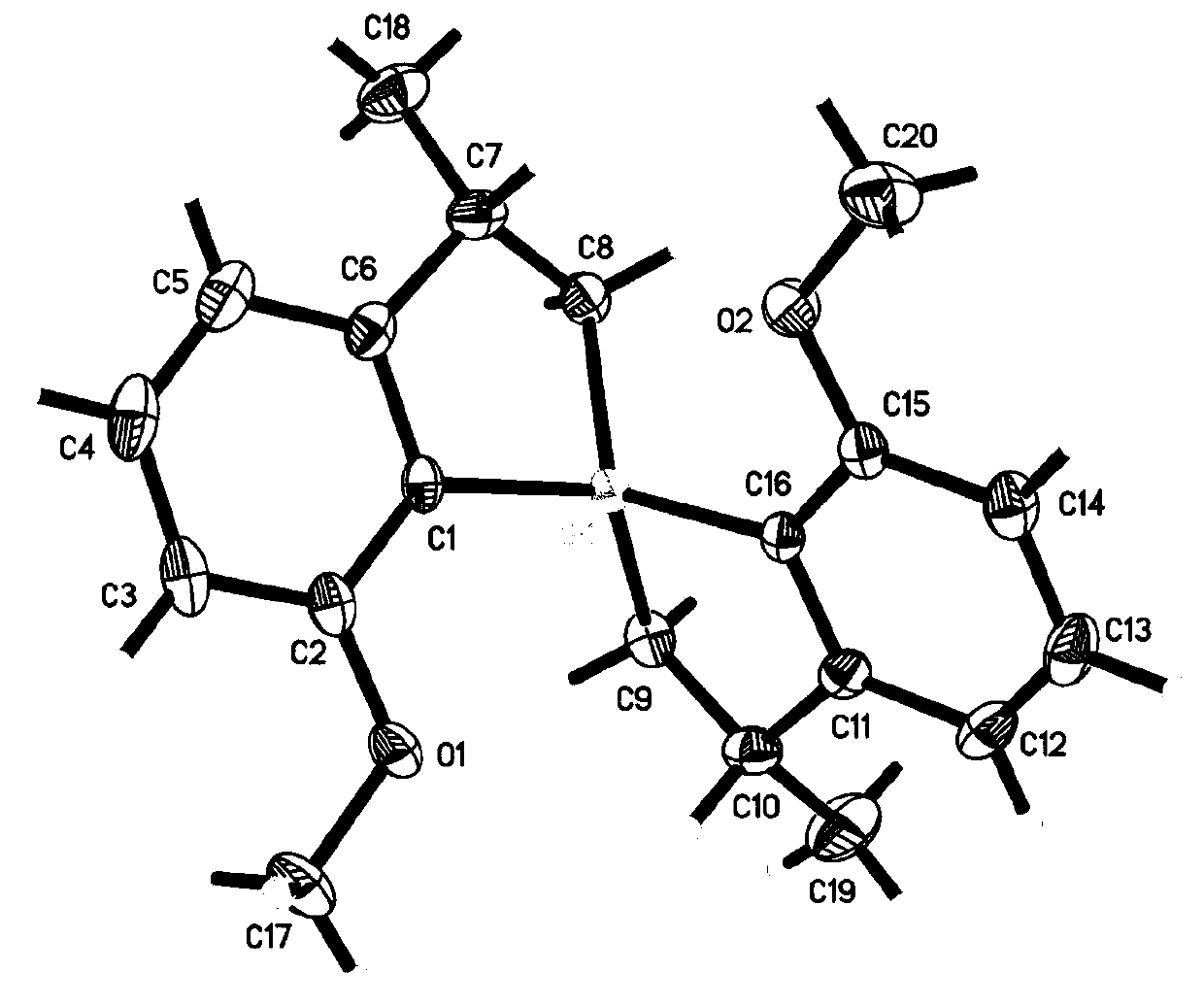
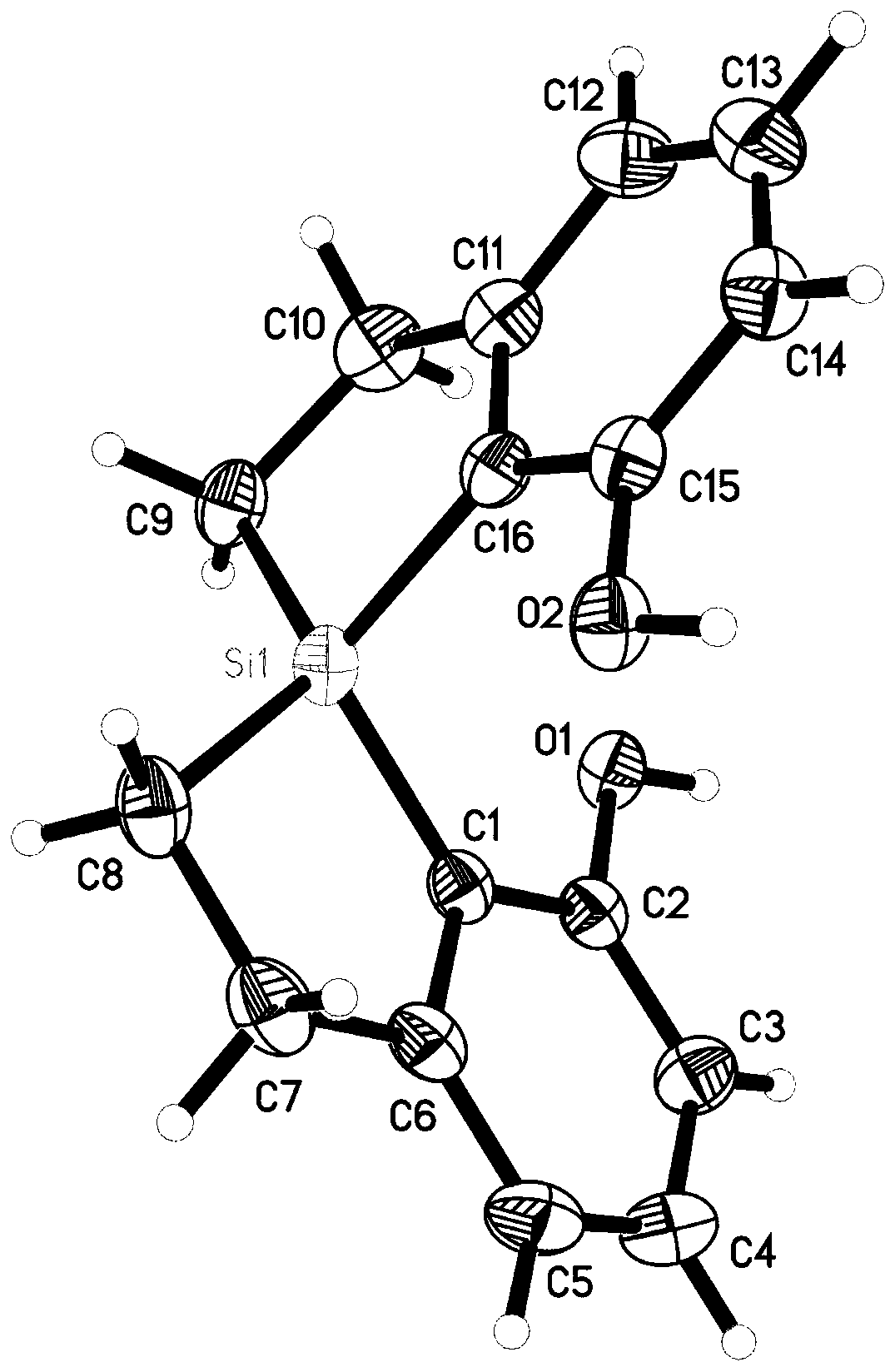
[0417] The crystals were subjected to X-ray diffraction detection, as attached figure 1 structure, consistent with the target structure.
[0418]
[0419]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com