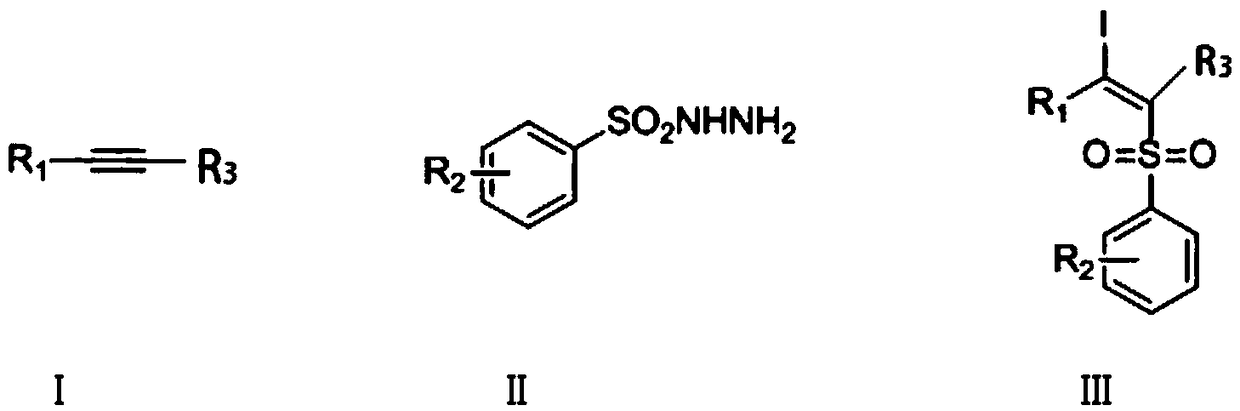
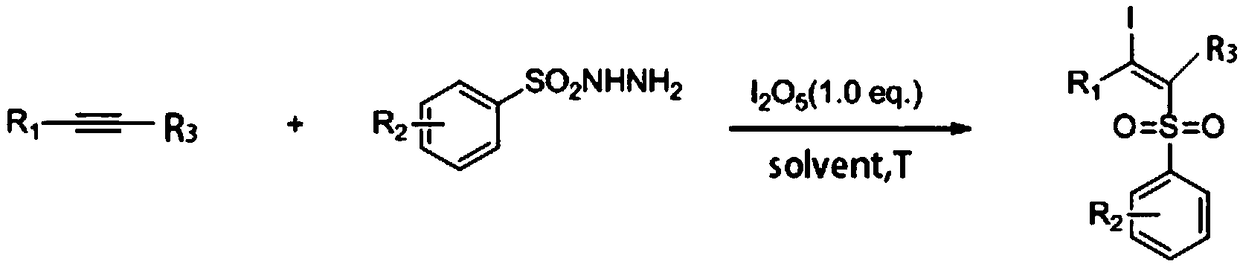
Synthetic method of beta-iodo-alkenyl sulfone compound
The technology of an iodoalkenyl sulfone and a synthesis method is applied in the field of synthesizing β-iodoalkenyl sulfones, which can solve the problems of potential safety hazards, lack of environmental friendliness, etc. Adaptability, mild effect of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
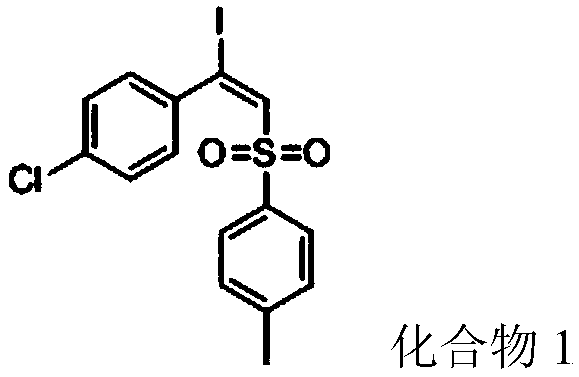
[0043] 4-Chlorophenylacetylene (68mg, 0.5mmol), p-toluenesulfonyl hydrazide (93mg, 0.5mmol), diiodine pentoxide (167mg, 0.5mmol) and solvent 1,4-dioxane 2mL were added to In a 10 mL sealed tube, heat and stir in an oil bath at 80°C for 8 h, stop heating after the reaction, cool the sealed tube to room temperature, and separate the crude product by column chromatography (the eluent is petroleum ether / ethyl acetate volume ratio 15:1 mixture), the product (E)-1-chloro-4-(1-iodo-2-p-toluenesulfonylvinyl)benzene (compound 1) 117.18 mg (yield 56%) was obtained.
[0044] Product characterization: white solid; mp=143-144°C; 1 H NMR (500MHz, CDCl 3) δ7.50(d, J=8.1Hz, 2H), 7.34(s, 1H), 7.29–7.22(m, 4H), 7.18(d, J=8.3Hz, 2H), 2.41(s,3H). 13 C NMR (125 MHz, CDCl 3 ) δ 144.88, 141.72, 138.06, 137.11, 135.87, 129.78, 129.09, 128.19, 127.85, 112.03, 21.65.
Embodiment 2
[0046]
[0047] 4-n-pentyloxyphenylacetylene (94mg, 0.5mmol), p-toluenesulfonyl hydrazide (186mg, 1.0mmol), diiodine pentoxide (167mg, 0.5mmol) and solvent 1,4-dioxane Add 2 mL into a 10 mL sealed tube, heat and stir in an oil bath at 80°C for 6 h, stop heating after the reaction, cool the sealed tube to room temperature, and separate the crude product by column chromatography (eluent is petroleum ether / ethyl acetate ester volume ratio of 15:1 mixture), to obtain the product (E)-1-n-pentyloxy-4-(1-iodo-2-p-toluenesulfonylvinyl)benzene (compound 2) 166.85 mg (yield 71%).
[0048] Product characterization: brown liquid; 1 H NMR (500MHz, CDCl 3) δ7.50 (d, J = 8.2Hz, 1H), 7.46–7.34 (m, 1H), 7.30–7.17 (m, 4H), 7.03 (d, J = 8.7Hz, 1H ),6.87–6.66(m,3H),3.95(dt,J=11.5,6.4Hz,2H),2.39(s,3H),1.78(dt,J=10.7,6.0Hz,2H),1.46–1.37( m, 4H), 0.94 (t, J = 7.1 Hz, 3H). 13 C NMR (126 MHz, CDCl 3 ) δ 160.46, 140.16, 137.53, 134.64, 131.56, 129.98, 129.63, 127.83, 113.69, 94.36, 68.14, 28.84, 2...
Embodiment 3
[0050]
[0051] Add 1-heptyne (48 mg, 0.5 mmol), p-toluenesulfonyl hydrazide (186 mg, 1.0 mmol), diiodine pentoxide (267 mg, 0.8 mmol) and 2 mL of solvent dimethyl sulfoxide into a 10 mL sealed tube, Heat and stir in an oil bath at 80°C for 6 hours, stop heating after the reaction is over, seal the tube and cool to room temperature, and separate the crude product by column chromatography (the eluent is a mixture of petroleum ether / ethyl acetate with a volume ratio of 15:1) liquid) to obtain 49.14 mg of product (E)-1-p-toluenesulfonyl-2-iodo-n-heptene (compound 3) (yield 26%).
[0052] Product characterization: brown liquid; 1 H NMR (500MHz, CDCl3) δ7.47 (d, J = 7.8Hz, 2H), 7.36 (s, 1H), 7.09 (d, J = 7.6Hz, 2H), 2.65-2.58 (m, 2H), 2.40 (s,3H), 1.69-1.61(m,2H), 1.38(s,4H), 0.94(s,3H). 13 C NMR (125MHz, CDCl3) δ 144.42, 140.94, 136.91, 129.56, 127.90, 114.96, 35.82, 31.53, 30.98, 22.55, 21.64, 14.06.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com