Synthesis method of bithiazole-4-yl disulfide derivative
A technology based on disulfides and synthesis methods, which is applied in the field of preparation of bithiazol-4-yl disulfide derivatives, can solve problems such as bad smell and instability of mercaptans, and ensure the health of operators and strong base The effect of universal applicability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
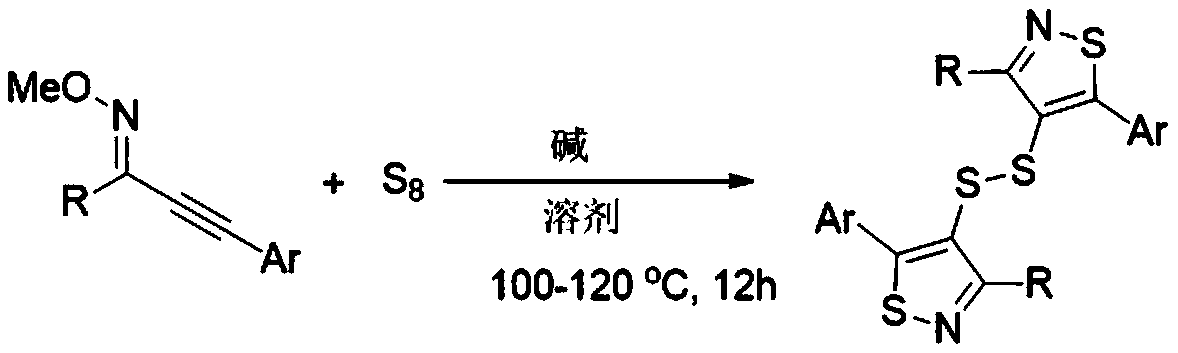
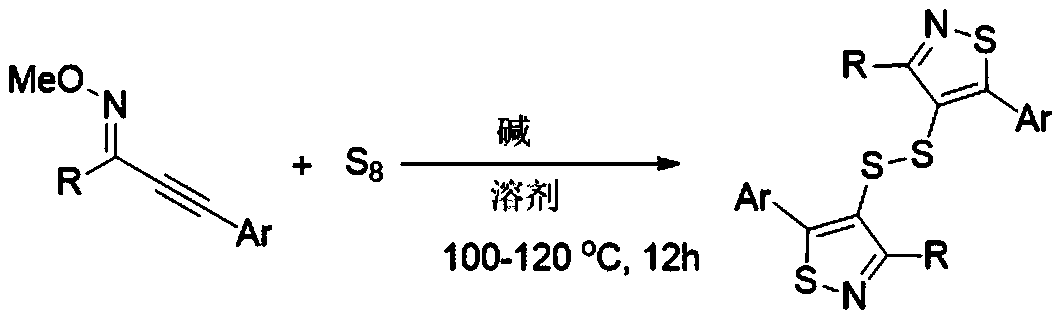
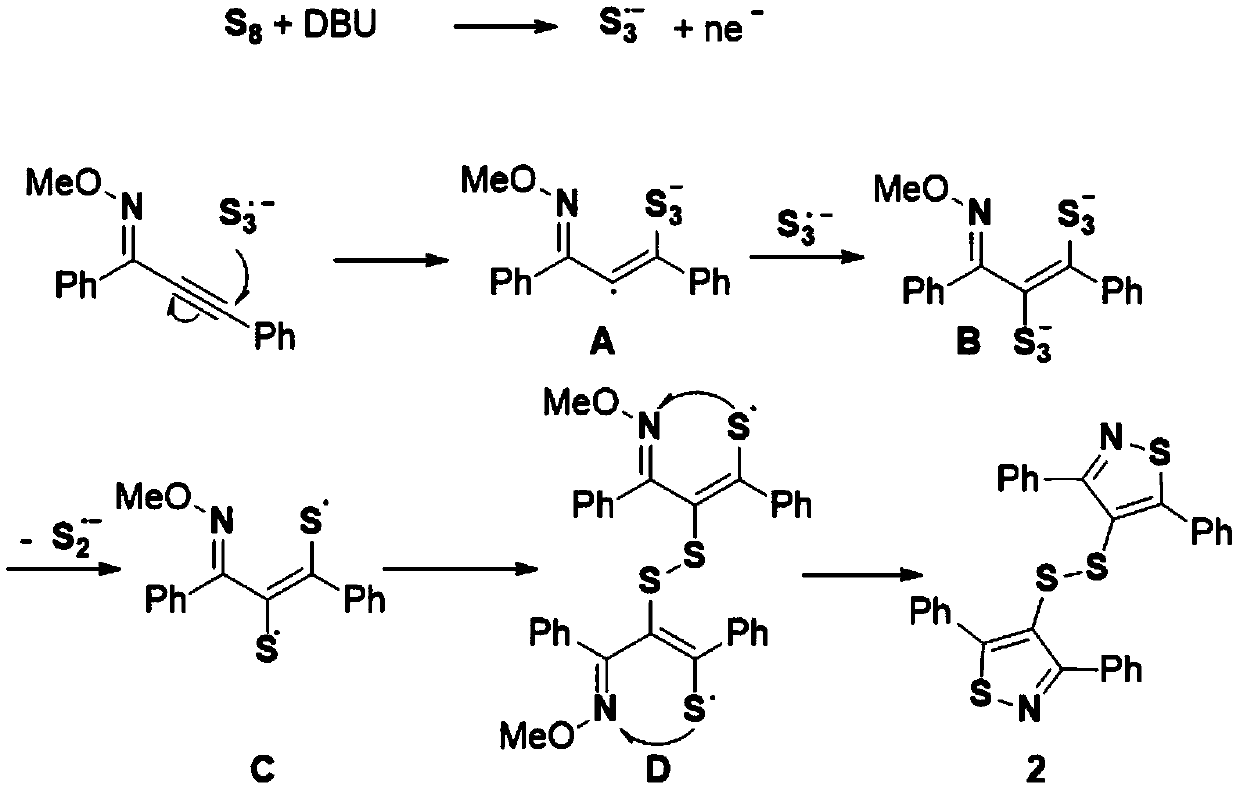
[0015] The invention discloses a synthesis method of bithiazol-4-yl disulfide derivatives, which comprises the following steps: using alkynyl oxime ether as a substrate, elemental sulfur as a sulfur source, and 1,8-diazabicycloundecone Carb-7-ene (DBU) as base, NMP-H 2 O(5:1,V:V) as solvent, stirred and reacted at 100℃~120℃ for 12 hours; the chemical reaction formula is as follows:
[0016]
[0017] The -R is phenyl, 4-methylphenyl, 2-methylphenyl, 4-ethylphenyl, 4-methoxyphenyl, 4-fluorophenyl, 4-chlorophenyl, 3 - one of chlorophenyl, 4-bromophenyl, 4-trifluoromethylphenyl, 2-naphthyl, 2-thienyl, cyclohexyl;
[0018] The -Ar is phenyl, 4-methylphenyl, 3-methylphenyl, 2-methylphenyl, 4-tert-butylphenyl, 4-methoxyphenyl, 4-fluorophenyl , 4-chlorophenyl, 4-trifluoromethylphenyl;
[0019] After the reaction was completed, after cooling, the reaction solution was filtered to obtain the filtrate, washed with saturated sodium chloride solution, extracted with ethyl acetate and...
specific Embodiment 1
[0020] Specific embodiment one: 47.0 milligrams (0.2mmol)-1,3-diphenylprop-2-yn-1-ketone o-methyl oxime, 102.4 milligrams (0.4mmol) elemental sulfur, 91.2 milligrams (0.6mmol) 1 , 8-diazabicycloundec-7-ene (DBU) was added to 2mL solvent NMP-H 2 O(5:1,V:V). The reaction was stirred at 120°C for 12 hours. Cool after the reaction, filter the reaction solution to obtain the filtrate, wash with saturated sodium chloride solution, extract with ethyl acetate and dry with anhydrous sodium sulfate, and use a rotary evaporator to remove the solvent from the filtrate to obtain a residue, which is passed through a silica gel column Use the eluent prepared by petroleum ether and ethyl acetate at a volume ratio of 30:1 for rinsing, collect the effluent according to the actual gradient, detect by TLC, combine the effluent containing the target product, and use rotary evaporation for the combined effluent Rotate the solvent to remove the solvent, and dry in vacuo to obtain 43.4 mg of yellow...
specific Embodiment 2
[0021] Specific embodiment two: 49.8 milligrams (0.2mmol) 1-phenyl-3-(p-tolyl) propyl group-2-yn-1-one oxime, 102.4 milligrams (0.4mmol) elemental sulfur, 91.2 milligrams (0.6mmol) )1,8-diazabicycloundec-7-ene (DBU) was added to 2mL solvent NMP-H 2 O(5:1,V:V). The reaction was stirred at 120°C for 12 hours. Cool after the reaction, filter the reaction solution to obtain the filtrate, wash with saturated sodium chloride solution, extract with ethyl acetate and dry with anhydrous sodium sulfate, and use a rotary evaporator to remove the solvent from the filtrate to obtain a residue, which is passed through a silica gel column Use the eluent prepared by petroleum ether and ethyl acetate at a volume ratio of 30:1 for rinsing, collect the effluent according to the actual gradient, detect by TLC, combine the effluent containing the target product, and use rotary evaporation for the combined effluent Rotate the solvent to remove the solvent, and dry in vacuo to obtain 47.4 mg of ye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com