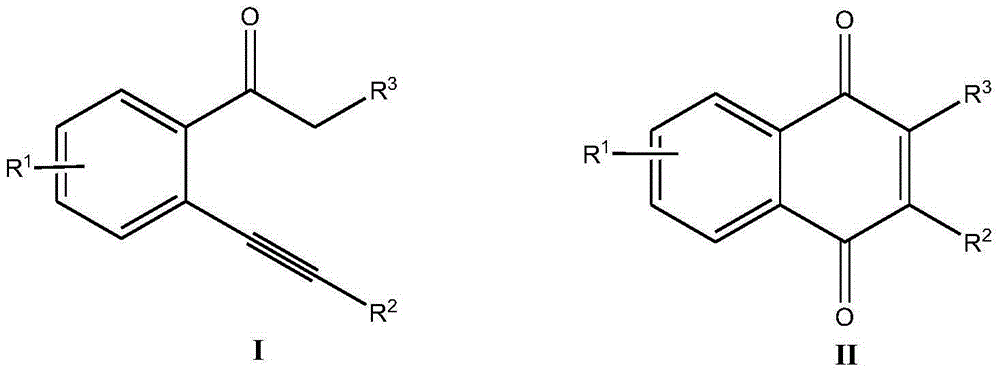
Synthetic method for 2-substituted-1,4-naphthoquinone derivatives
A technology of derivatives and naphthoquinones, applied in the field of organic compound synthesis, can solve the problems of complex post-treatment, long reaction time, and low reaction selectivity, and achieve good substrate universality, strong substrate universality, The effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
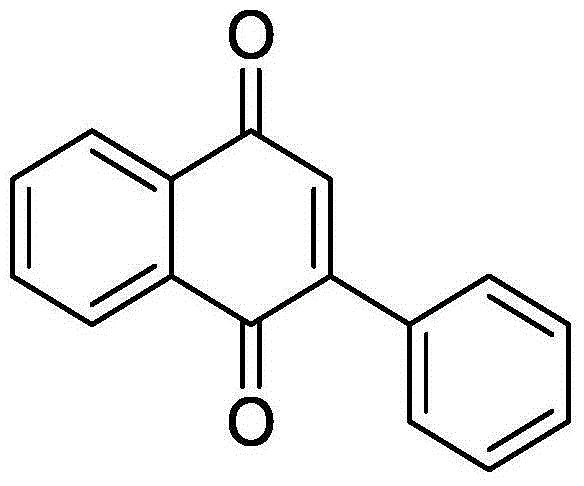
[0030] 44.0mg (0.2mmol) o-phenylethynyl acetophenone, 7.22mg (0.02mmol) bistrifluoromethanesulfonate copper (Cu (OTf) 2 ), 3.82mg (0.02mmol) CuI were added to a 10mL round bottom flask, and then 2mL DMSO was added as solvent and oxidant. Next, magnetically stir at 140° C. for 6 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) As an eluent, collect the eluent containing the product, and evaporate the solvent from the eluent to obtain the pure product 2-phenyl-1,4-naphthoquinone. The material was a yellow solid in 92% yield.
[0031] Characterization data: 1 H NMR (CDCl 3 ,500MHz): δ8.20-8.18(m, 1H), 8.13-8.12(m, 1H), 7.60-7.58(m, 2H), 7.50-7.48(m, 3H), 7.09(s, 1H); 13 C NMR (CDCl 3 , 125MHz): δ185.0, 184.3, 148.1, 135.2, 133.8, 133.8, 133.4, 132.5, 132.1, 130.0, 129.4, 128.4, 127.0, 125.9.
Embodiment 2
[0033]
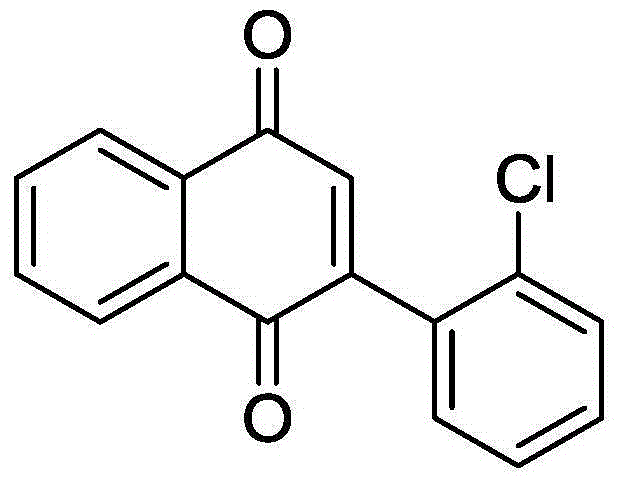
[0034] 50.9mg (0.2mmol) 2-o-chlorophenylethynyl acetophenone, 3.61mg (0.01mmol) Cu (OTf) 2 , 1.91mg (0.01mmol) CuI was added to a 10mL round bottom flask, and then 2mL DMSO was added as a solvent and an oxidizing agent. Next, magnetically stir at 120° C. for 10 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) As an eluent, collect the eluent containing the product, and evaporate the solvent from the eluent to obtain the pure product 2-(2-chlorophenyl)-1,4-naphthoquinone. The material was a yellow solid in 81% yield.
[0035] Characterization data: 1 H NMR (CDCl 3 ,500MHz): δ=8.20-8.16(m, 2H), 7.82-7.81(m, 2H), 7.52(dd, J1=7.5Hz, J2=1Hz, 1H), 7.44-7.38(m, 2H), 7.31 (dd, J1=7.5Hz, J2=2Hz, 1H), 7.02(s, 1H); 13 C NMR (CDCl 3 , 125MHz): δ184.9, 183.1, 148.2, 137.4, 134.0, 133.9, 133.2, 133.2, 132.1, 130.6, 130.6, 129.8, 127.1, 126.8, 126.2.
Embodiment 3
[0037]
[0038] 46.8mg (0.2mmol) 2-o-methylphenylethynyl acetophenone, 7.22mg (0.02mmol) Cu (OTf) 2 , 3.82mg (0.02mmol) CuI was added to a 10mL round bottom flask, and then 4mL DMSO was added as solvent and oxidant. Then, magnetically stir at 80° C. for 10 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) As an eluent, collect the eluent containing the product, and evaporate the solvent from the eluent to obtain the pure product 2-(2-methylphenyl)-1,4-naphthoquinone. The material was a yellow solid in 83% yield.
[0039] Characterization data: 1 H NMR (CDCl 3 ,500MHz): δ8.19-8.15(m, 2H), 7.81-7.79(m, 2H), 7.39-7.36(m, 1H), 7.32-7.27(m, 2H), 7.20(dd, J1=7.5Hz , J2=1Hz, 1H), 6.95(s, 1H), 2.25(s, 3H); 13 C NMR (CDCl 3 , 125MHz): δ185.2, 184.0, 150.7, 136.9, 136.2, 133.9, 133.9, 132.3, 132.2, 130.4, 129.4, 129.2, 127.0, 126.1, 125.8, 20.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com