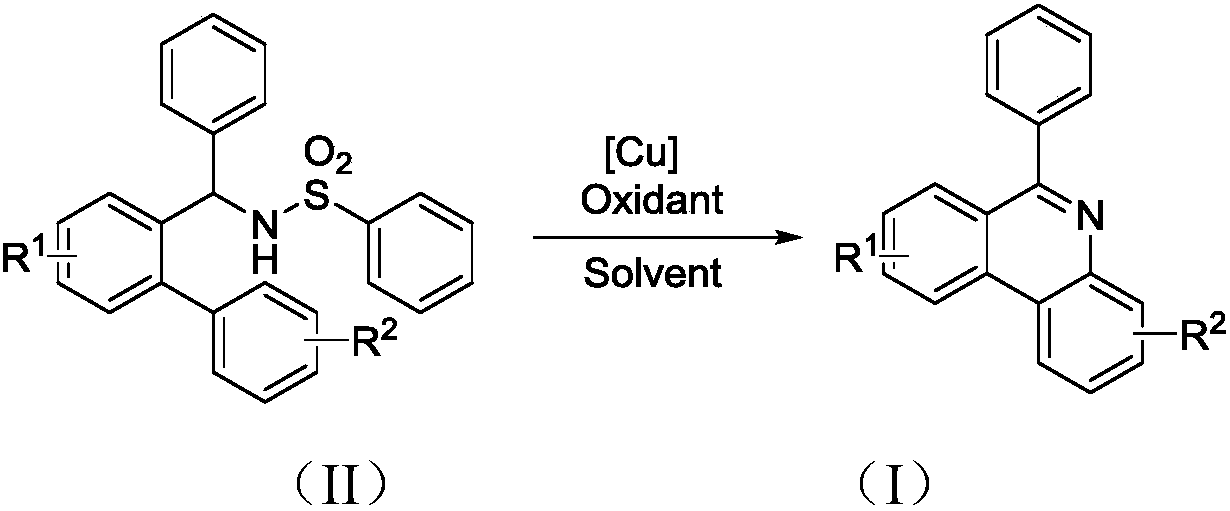
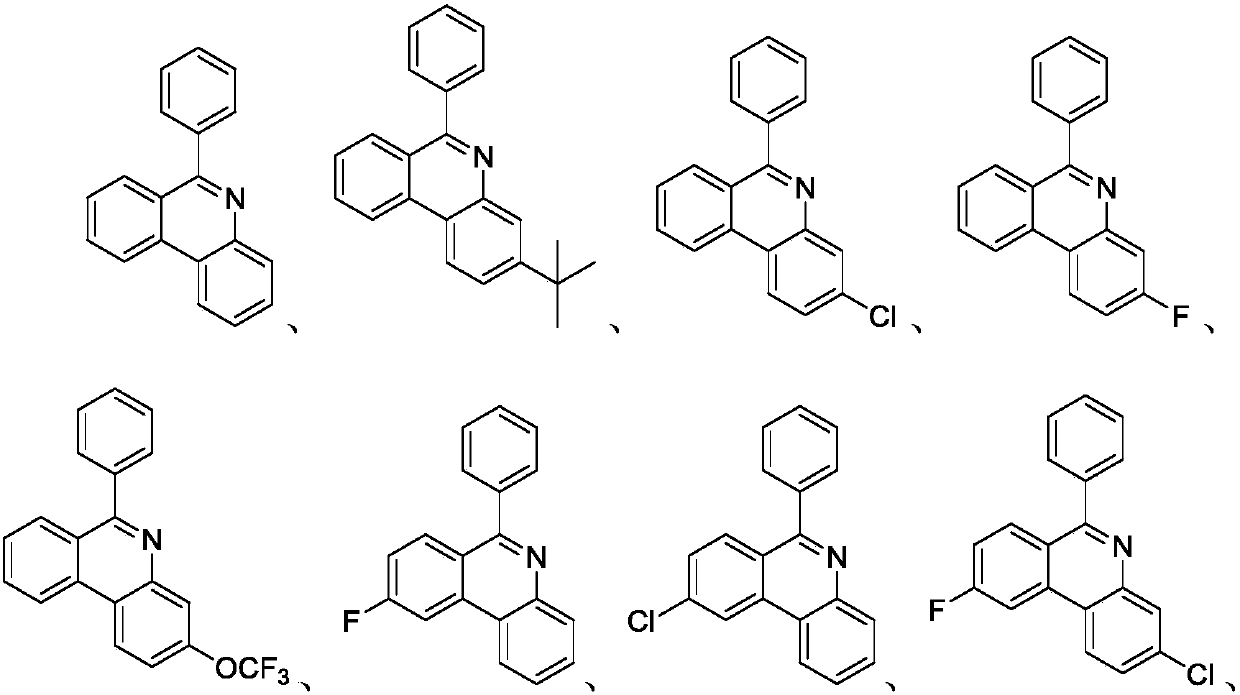
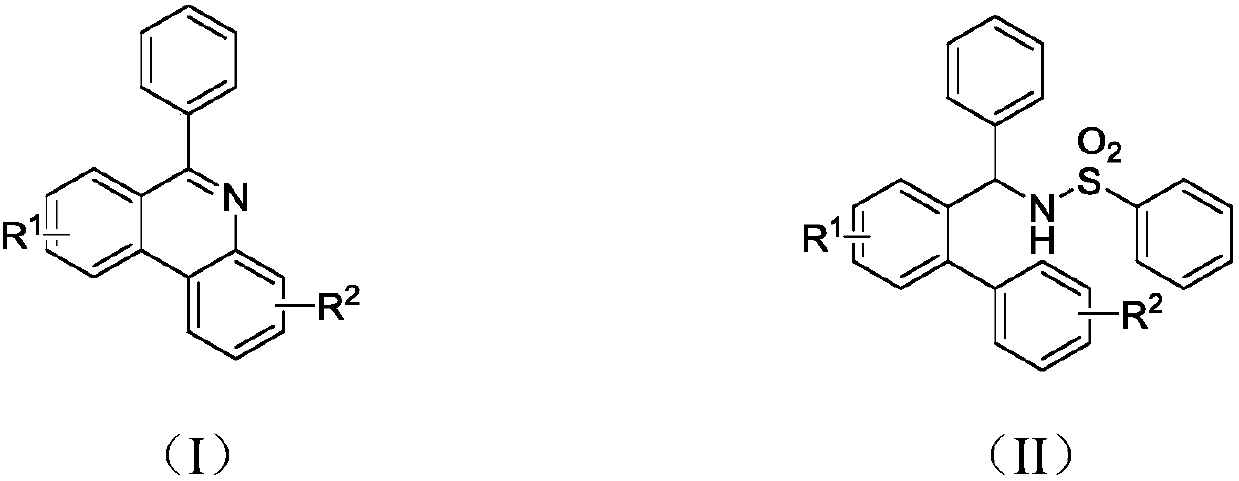Preparation method of 6-phenyl phenanthridine compound
A technology for phenylphenanthridine and compounds, which is applied in the field of preparation of organic compounds, can solve the problems of expensive visible light or thermal energy catalysts, expensive catalysts, harsh reaction conditions, etc., and achieves low-cost and easy-to-obtain catalysts and universal substrates. The effect of strong performance and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Add 119.7mg (0.3mmol) N-[1-phenyl-1'-biphenyl]benzenesulfonamide, 212.6mg (0.6mmol) Selectfluor and 5.7mg (0.03mmol) CuI into a 25mL thick-walled pressure-resistant tube, Acetonitrile was used as solvent, and the dosage was 3 mL. Next, it was magnetically stirred at 70° C. for 12 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 8mL of dichloromethane and 8mL of water to extract simultaneously, extract 3 times, get the organic layer containing dichloromethane and target product each time, i.e. the extract, and in the last Add 239.4 mg of column chromatography silica gel (100-200 mesh) to the extract, remove the solvent by distillation under reduced pressure (vacuum degree is 0.08kPa), and separate the residue by column chromatography. The volume ratio of petroleum ether and ethyl acetate is 10 : 1 mixed solution was eluted as eluent, the eluate containing target product was collected, and the solvent was evaporated to obtain a ...
Embodiment 2
[0028]
[0029] 136.5 mg (0.3 mmol) N-[1-phenyl-1'-biphenyl (4'-tert-butyl)] benzenesulfonamide, 204.3 mg (0.9 mmol) DDQ and 6.5 mg (0.045 mmol) CuBr were added to In a 25mL thick-walled pressure-resistant tube, acetone is used as a solvent, and the dosage is 3mL. Next, it was magnetically stirred at 100° C. for 24 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 12mL ethyl acetate and 8mL water to extract at the same time, extract 3 times, get the organic layer containing ethyl acetate and the target product each time, i.e. the extract, and in the last Add 273 mg of column chromatography silica gel (100-200 mesh) to the extract, remove the solvent by distillation under reduced pressure (vacuum degree is 0.08kPa), and separate the residue by column chromatography. The volume ratio of petroleum ether and ethyl acetate is 10: The mixed solution of 1 was eluted as an eluent, and the eluate containing the target product was collected, and the sol...
Embodiment 3
[0032]
[0033] Add 129.9 mg (0.3 mmol) N-[1-phenyl-1'-biphenyl(4'-chloro)]benzenesulfonamide, 553.3 mg (0.9 mmol) Oxone and 8.6 mg (0.045 mmol) CuI to a 25 mL thick In the wall pressure tube, toluene is used as solvent, and the dosage is 0.9mL. Next, magnetic stirring was performed at 120° C. for 36 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 3mL dichloromethane and 8mL water to extract simultaneously, extract 3 times, get the organic layer containing dichloromethane and target product at each time, i.e. the extract, and in the last Add 259.8 mg of column chromatography silica gel (100-200 mesh) to the extract, remove the solvent by distillation under reduced pressure (vacuum degree is 0.08kPa), and separate the residue by column chromatography. The volume ratio of petroleum ether to ethyl acetate is 10 : The mixed solution of 1 was eluted as the eluent, and the eluate containing the target product was collected, and the solvent was eva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com