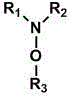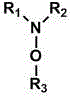Patents
Literature
464results about "Quinone preparation by oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
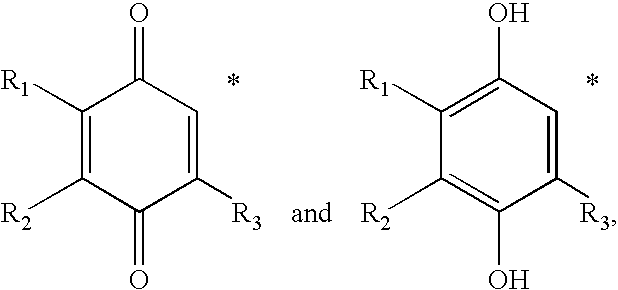
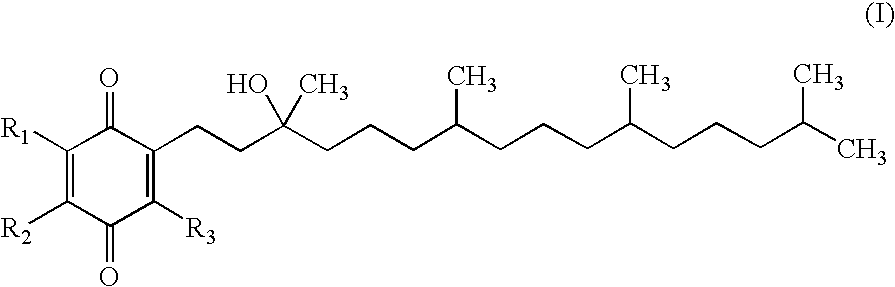
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
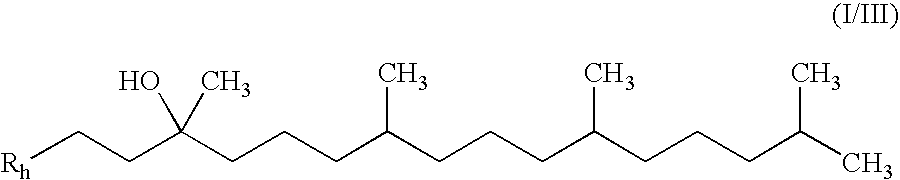
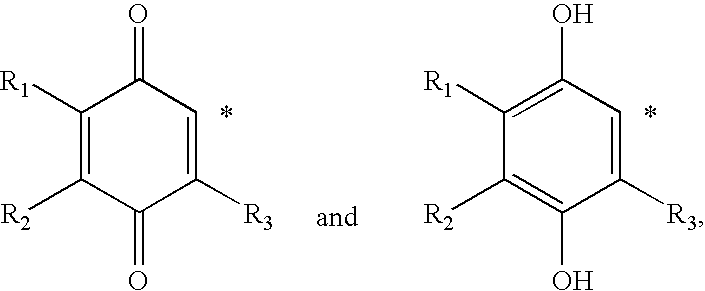
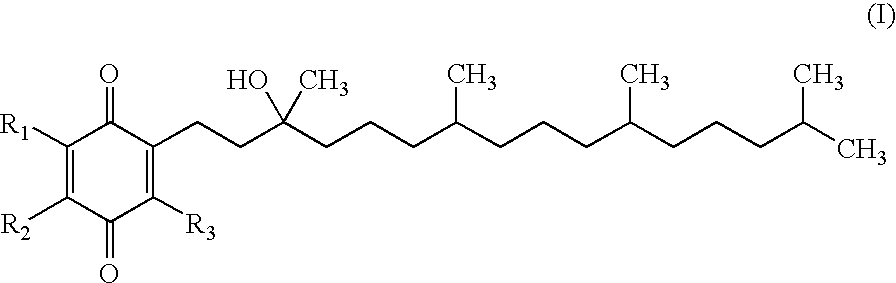
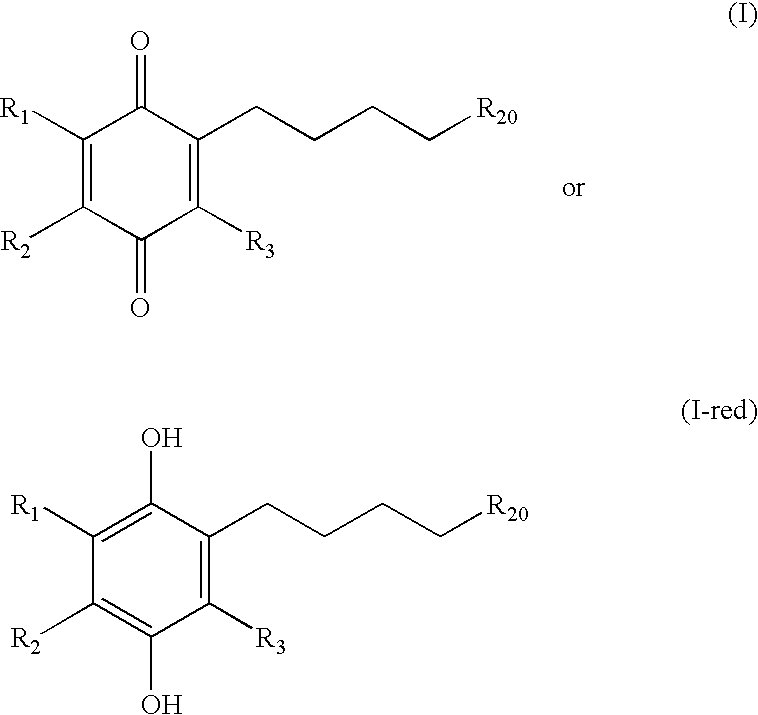
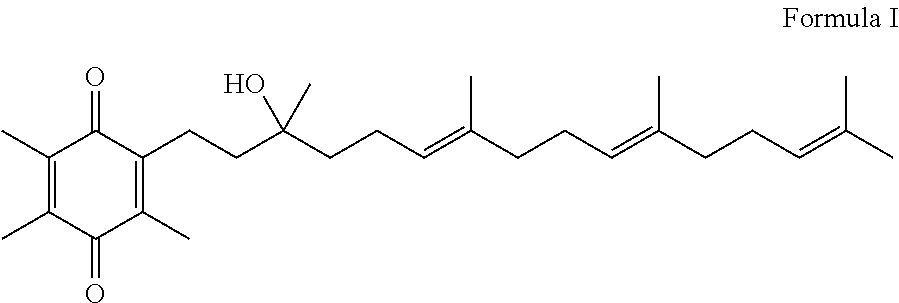
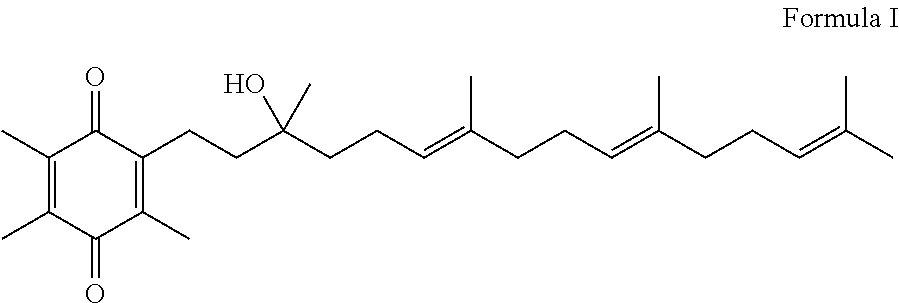
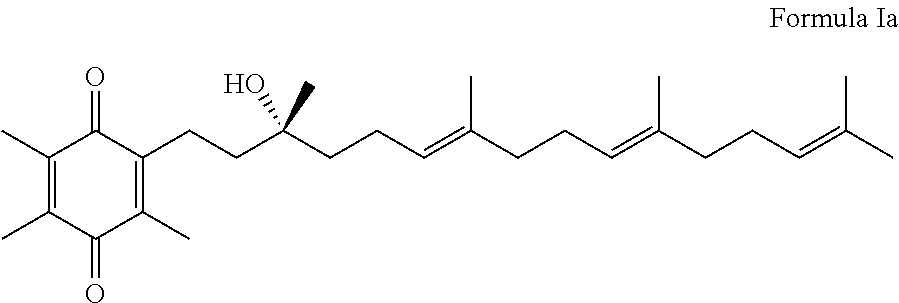
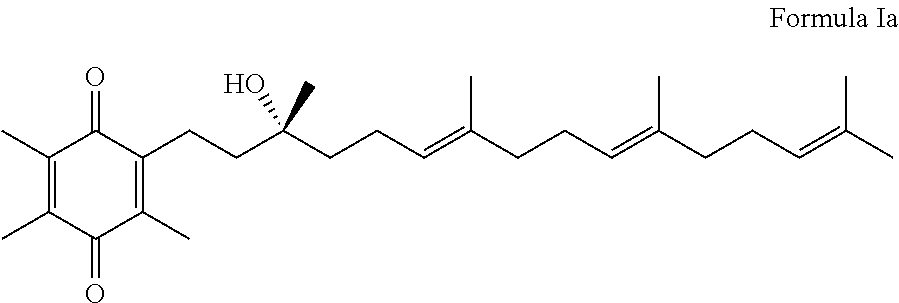
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Tail variants of redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
ActiveUS7432305B2Easy to modifyReduce severityAntibacterial agentsBiocideKearn sayre syndromeMitochondrial myopathy
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed.
Owner:PTC THERAPEUTICS INC
Tail variants of redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
ActiveUS20070072943A1Lower Level RequirementsExercise toleranceAntibacterial agentsBiocideKearn sayre syndromeFriedreichs ataxia
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed.
Owner:PTC THERAPEUTICS INC
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
ActiveUS20100222436A1Reduce severityReduce in quantityBiocideSenses disorderDiseaseKearn sayre syndrome
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
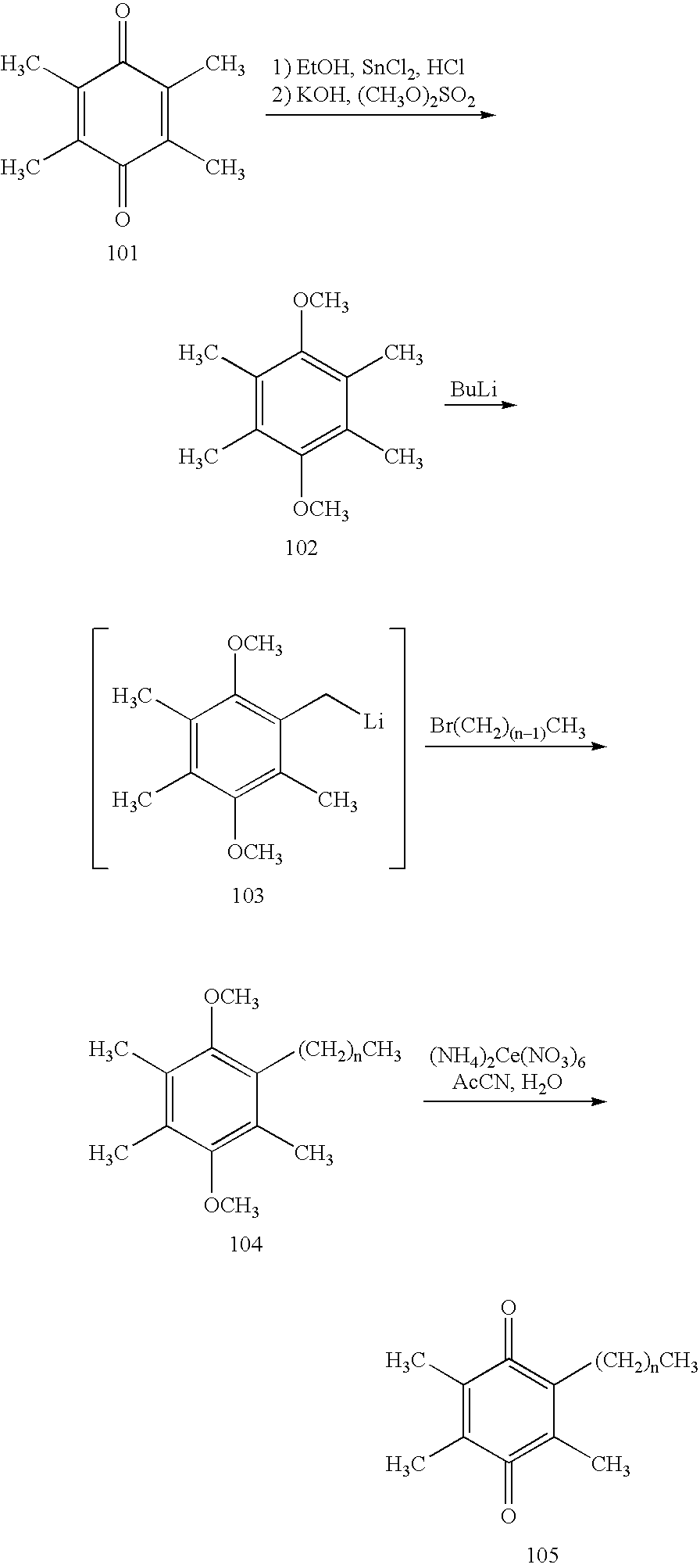
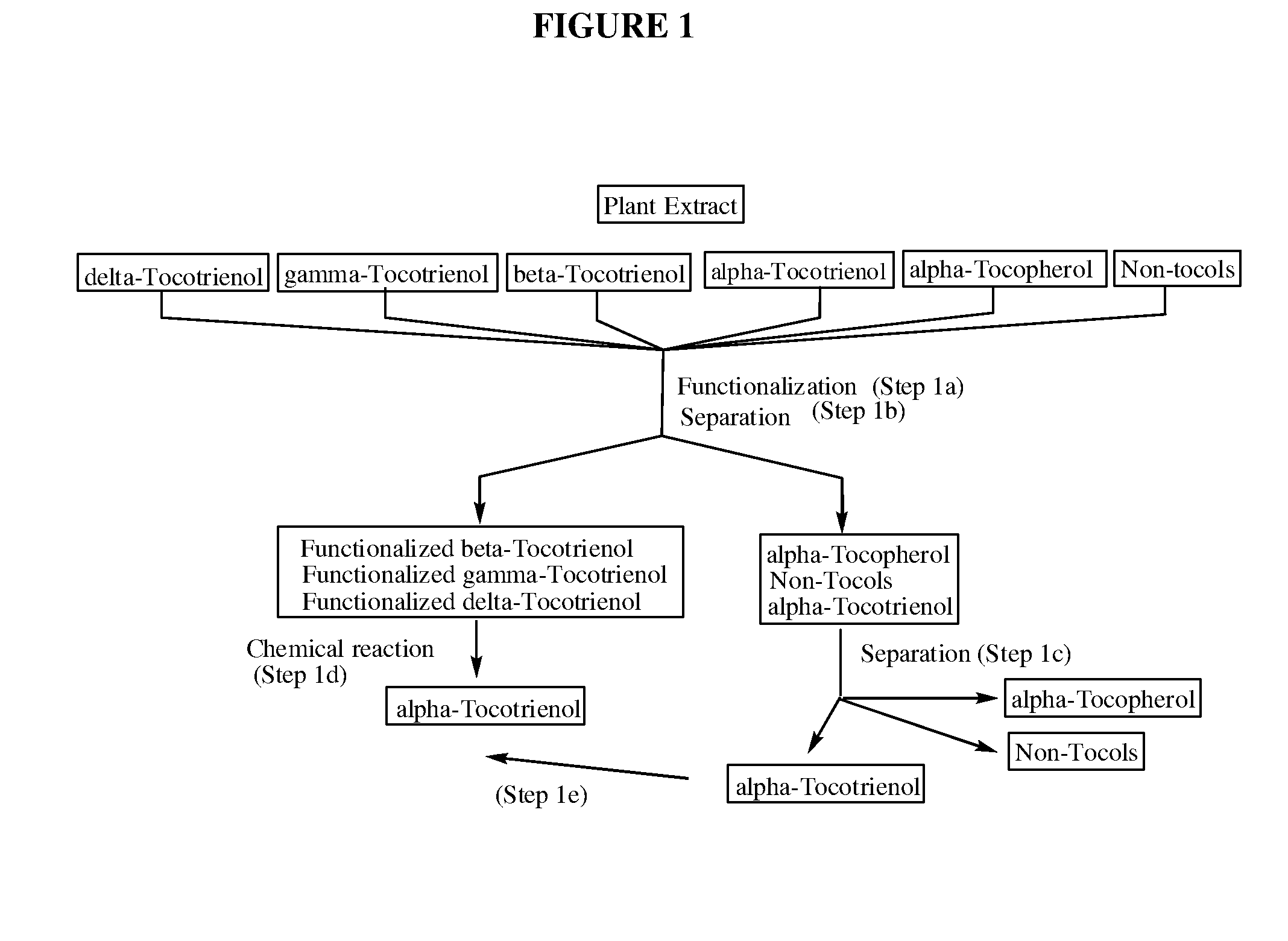
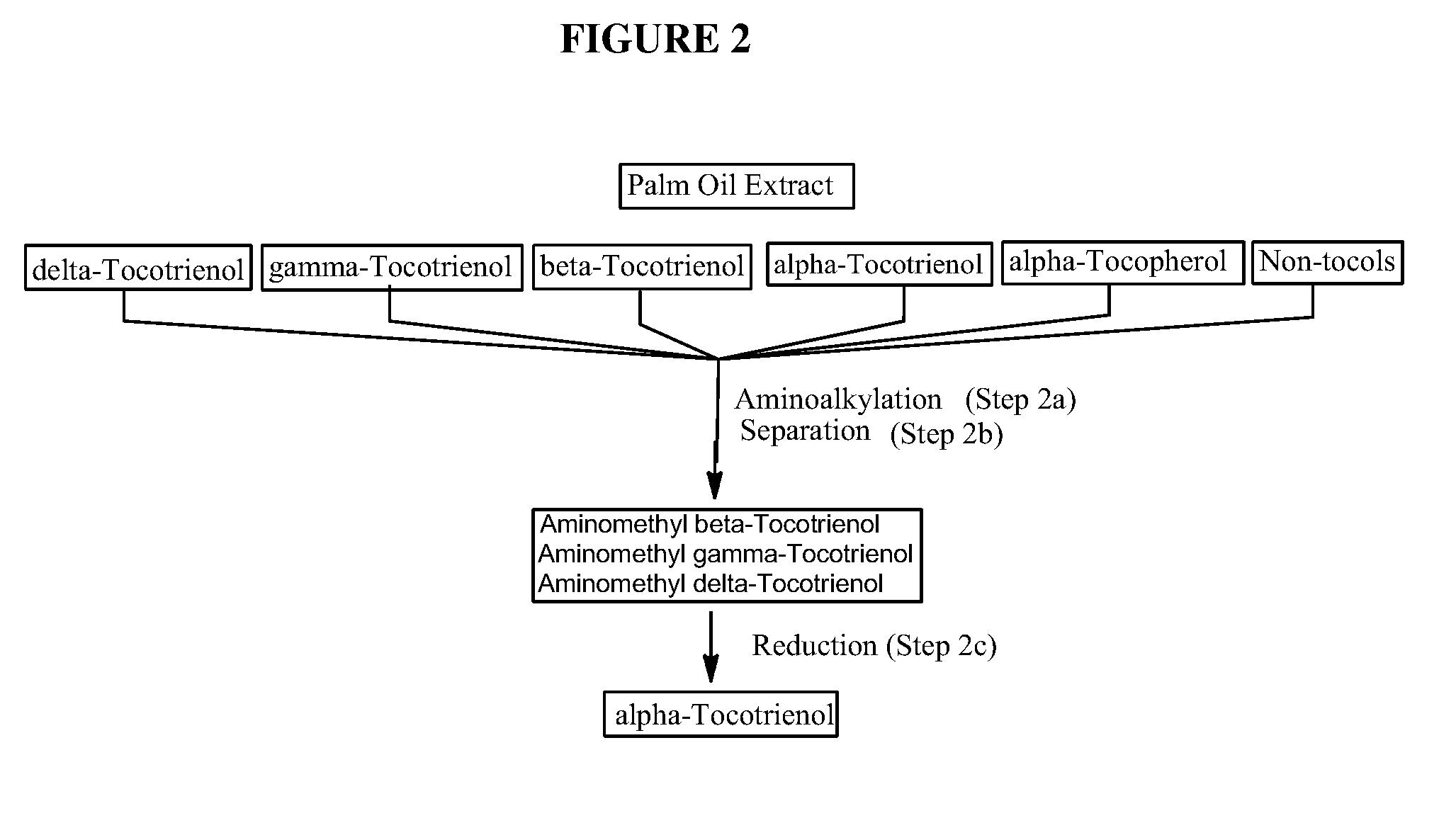
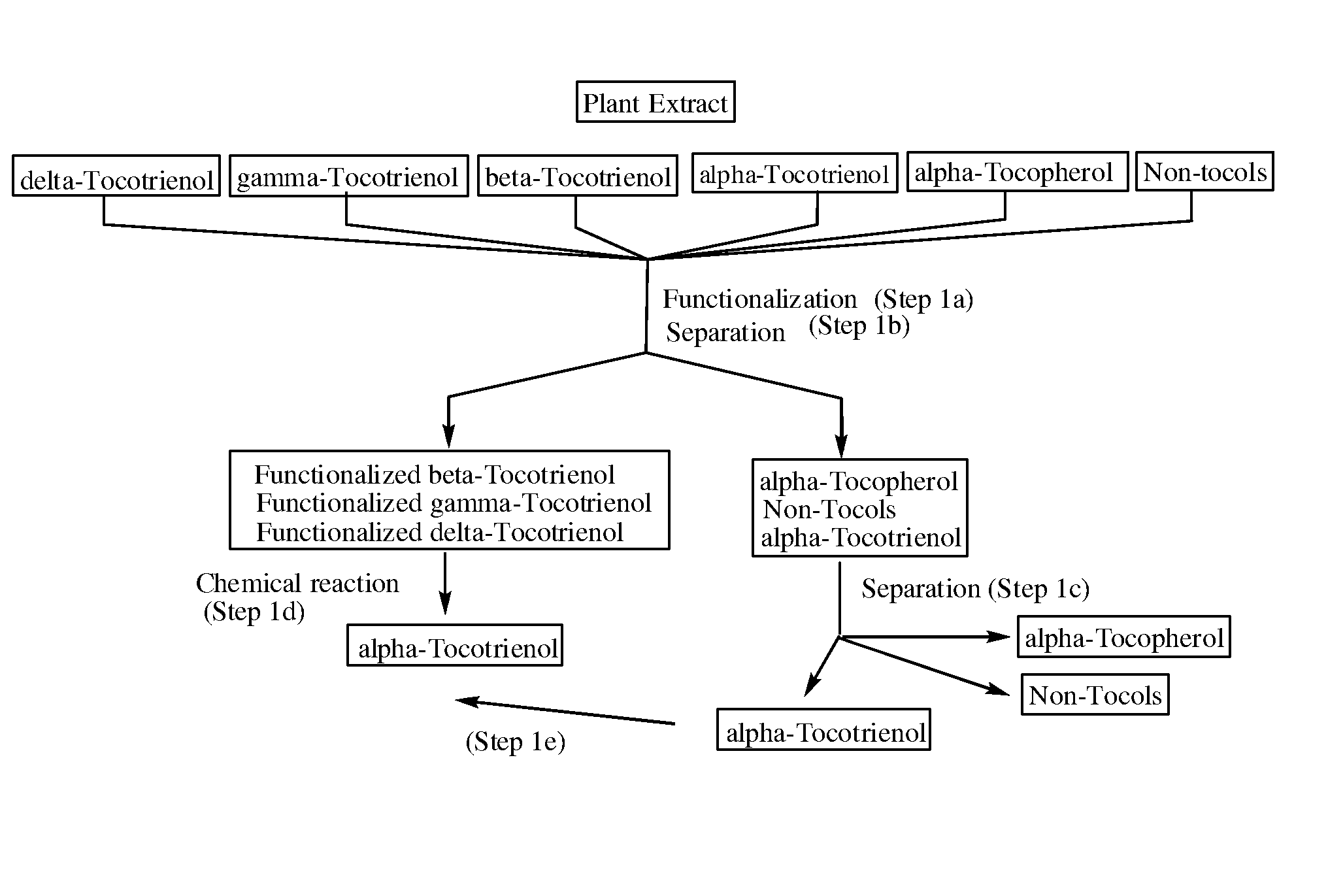
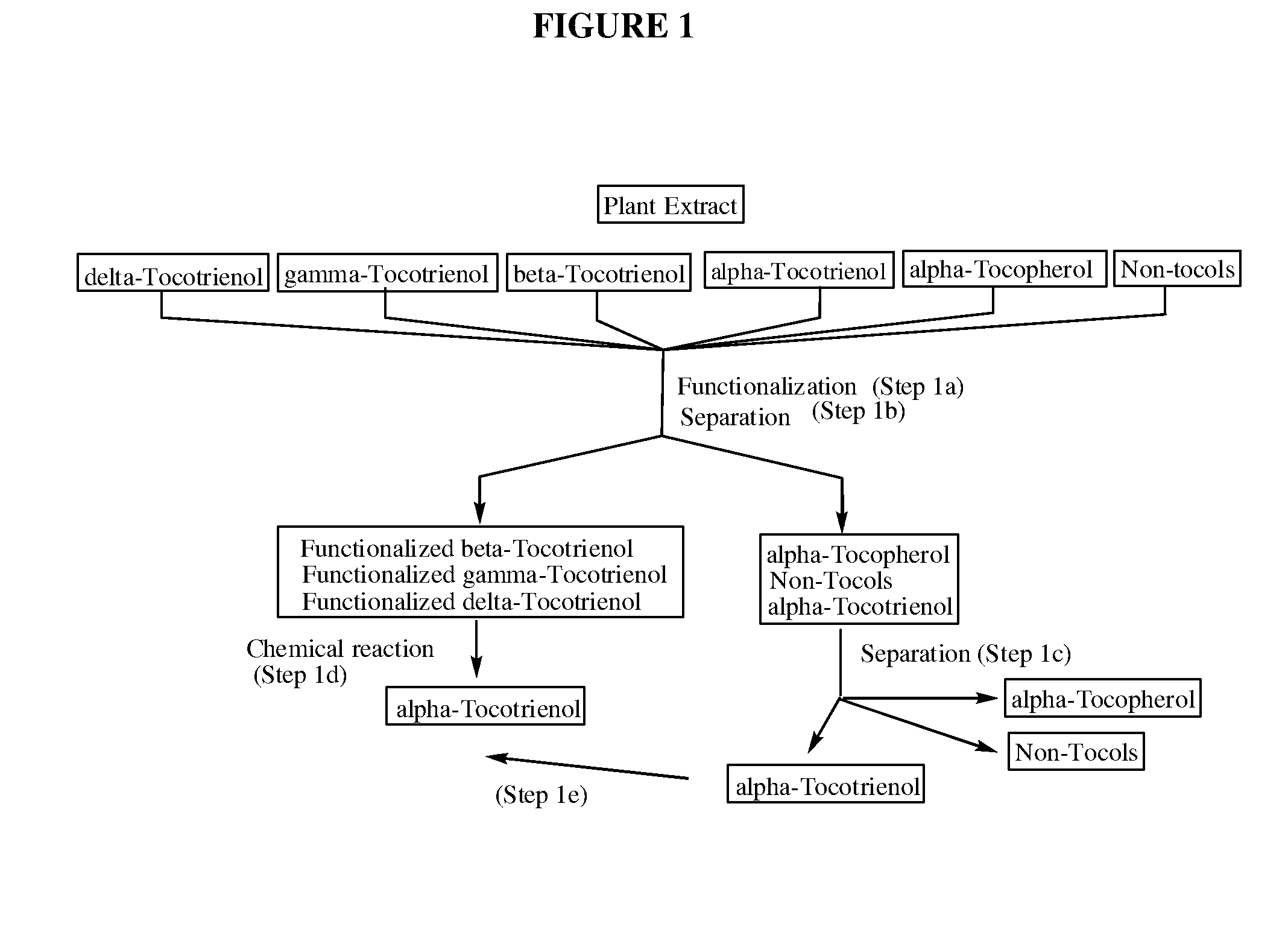
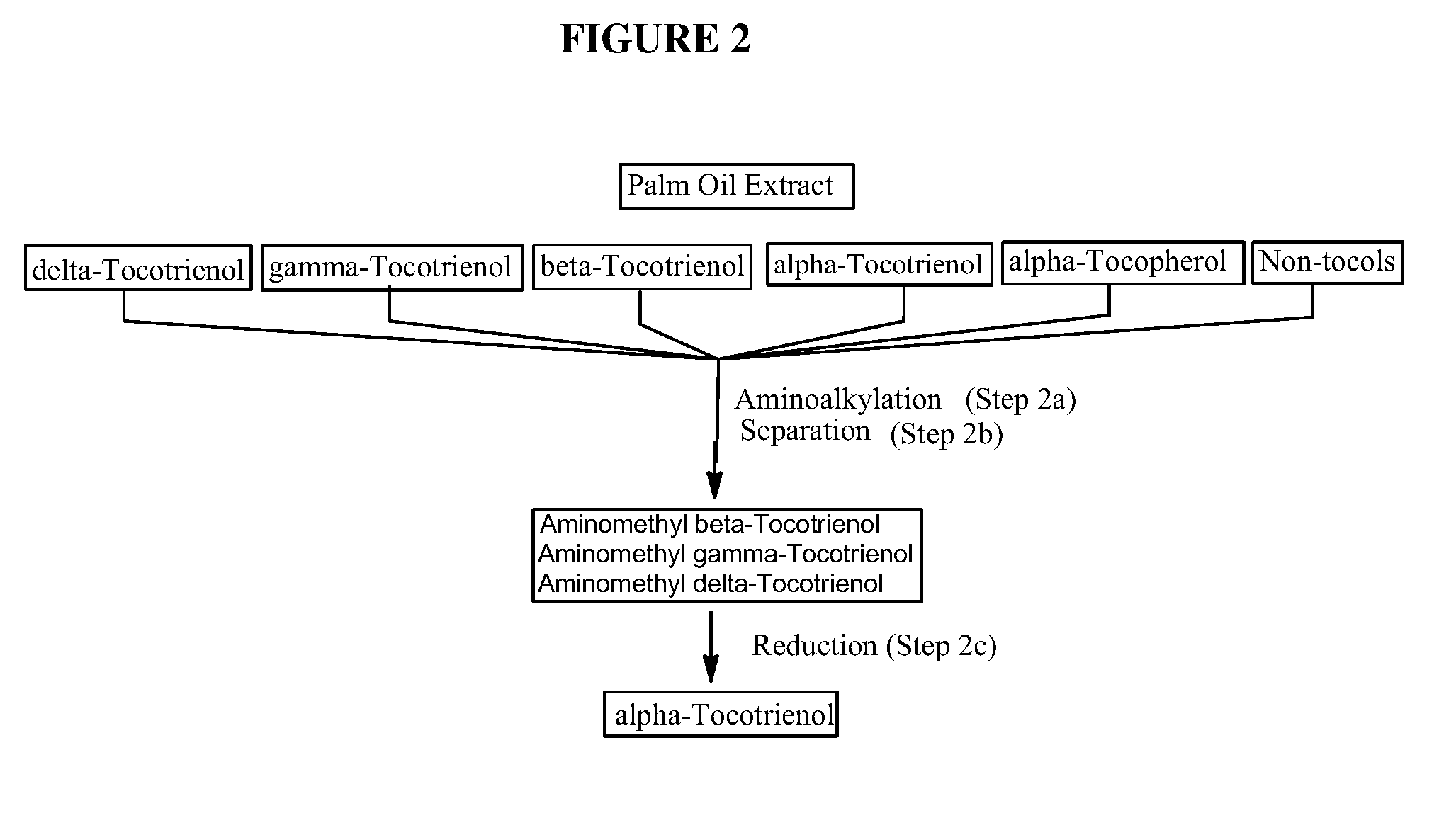
Process for the production of alpha-tocotrienol and derivatives
ActiveUS8106223B2High purityImprove solubilityQuinone preparation by oxidationSource materialAlpha-Tocotrienol
The invention discloses novel processes for production, enrichment and / or isolation of alpha-tocotrienol from source material comprising at least one non-alpha-tocotrienol, such as natural extracts comprising mixed tocotrienols.
Owner:PTC THERAPEUTICS INC
Method for preparing 2-amylanthraquinone by two-step method
ActiveCN107602368ALow costReduce pollutionQuinone preparation by oxidationHydrocarbonsAlkyl transferAnthracene
The invention discloses a method for preparing 2-amylanthraquinone by a two-step method. The method comprises a first step alkylation process (anthracene and isoamylene perform alkylation reaction under the effect of catalysts to produce 2-amylanthraquinone) and a second step oxidization process (2-amylanthraquinone takes oxidization reaction under the effects of catalysts to produce 2-amylanthraquinone; solid-state catalysts are selected to take multi-phase reaction; catalysts can be cyclically used through regeneration). The atom utilization rate reaches 100 percent; the raw material utilization rate is improved; the waste pollution is reduced; the anthracene and the isoamylene are used as raw materials; the solid-catalysts are preferably selected to be used as solid catalysts; the repeated cyclic utilization is realized; the production cost of the 2-amylanthraquinone is obviously reduced. The 2-amylanthraquinone is prepared by the two-step method; the method is simple; the reactionconditions are mild; the production pollution is obviously reduced; the oxygen gas is preferably selected to be used as oxidizing agents, so that the cost is lower; the application prospects are wider.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for the production of alpha-tocotrienol and derivatives
ActiveUS20100105930A1Need of intensive and expensiveEconomical commercial processQuinone preparation by oxidationChemistrySource material
The invention discloses novel processes for production, enrichment and / or isolation of alpha-tocotrienol from source material comprising at least one non-alpha-tocotrienol, such as natural extracts comprising mixed tocotrienols.
Owner:PTC THERAPEUTICS INC
Technology for preparing 2-ethylanthraquinone
InactiveCN107746372AReduce the difficulty of productionLow costMolecular sieve catalystQuinone preparation by oxidationMolecular sieveAnthracene
The invention discloses a technology for preparing 2-ethylanthraquinone from anthracene and ethylene. The technology comprises the following steps: alkylating anthracene and ethylene by using a transition metal oxide supported MWW type molecular sieve catalyst to prepare 2-ethylanthracence; and oxidizing the 2-ethylanthracence by using a molybdena supported Y type molecular sieve catalyst to prepare the 2-ethylanthraquinone. The technology has the advantages of facilitation of the proceeding of the reactions, low cost and high yield.
Owner:QINZHOU UNIV
Electrochemically active agents for ph modulation in biological buffers
ActiveUS20170008825A1ReactivityReduced responseOrganic compound preparationQuinone preparation by oxidationAnalyteActive agent
Device and methods for use in a biosensor comprising a multisite array of test sites, the device and methods being useful for modulating the binding interactions between a (biomolecular) probe or detection agent and an analyte of interest by modulating the pH or ionic gradient near the electrodes in such biosensor. An electrochemically active agent that is suitable for use in biological buffers for changing the pH of the biological buffers. Method for changing the pH of biological buffers using the electrochemically active agents. The methods of modulating the binding interactions provided in a biosensor, analytic methods for more accurately controlling and measuring the pH or ionic gradient near the electrodes in such biosensor, and analytic methods for more accurately measuring an analyte of interest in a biological sample.
Owner:ROBERT BOSCH GMBH
Practical, cost-effective synthesis of CoQ10
InactiveUS6852895B2Convenient and efficient and inexpensive entryEfficient and inexpensive methodOrganic compound preparationQuinone preparation by oxidationCombinatorial chemistry
Owner:ZYMES
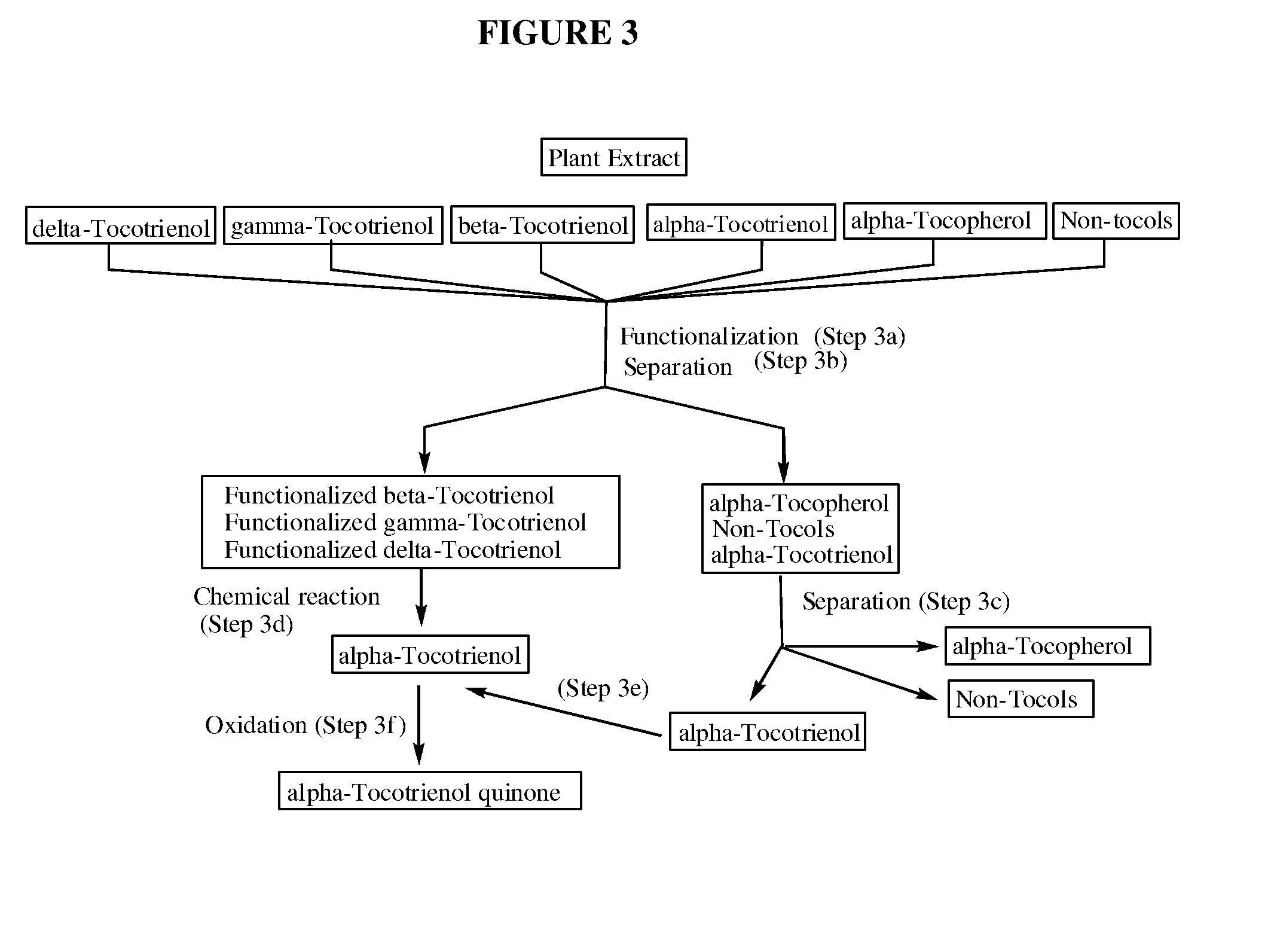
Methods for selective oxidation of alpha tocotrienol in the presence of non-alpha tocotrienols
ActiveUS20140249332A1Shorten the progressReduce or eliminate either a diseaseSenses disorderOrganic compound preparationAlpha-TocotrienolOxidizing agent
A method of producing alpha-tocotrienol quinone or a stereoisomer thereof, the method comprising selective opening of alpha-tocotrienol chroman to alpha-tocotrienol quinone in the presence of non-alpha tocotrienol chromans by oxidizing alpha-tocotrienol with a metal salt oxidizing agent, wherein the stoichiometric ratio of metal salt oxidizing agent / alpha-tocotrienol is at least 4:1 and wherein said metal oxidizing agent is added in sequential additions, in order to reduce oxidation of any amounts of non-alpha tocotrienol chromans that might have been present in the starting alpha-tocotrienol chroman material. This process uses conditions favoring oxidation rates of the alpha tocotrienol chroman vs the non-alpha tocotrienol chromans.
Owner:PTC THERAPEUTICS INC
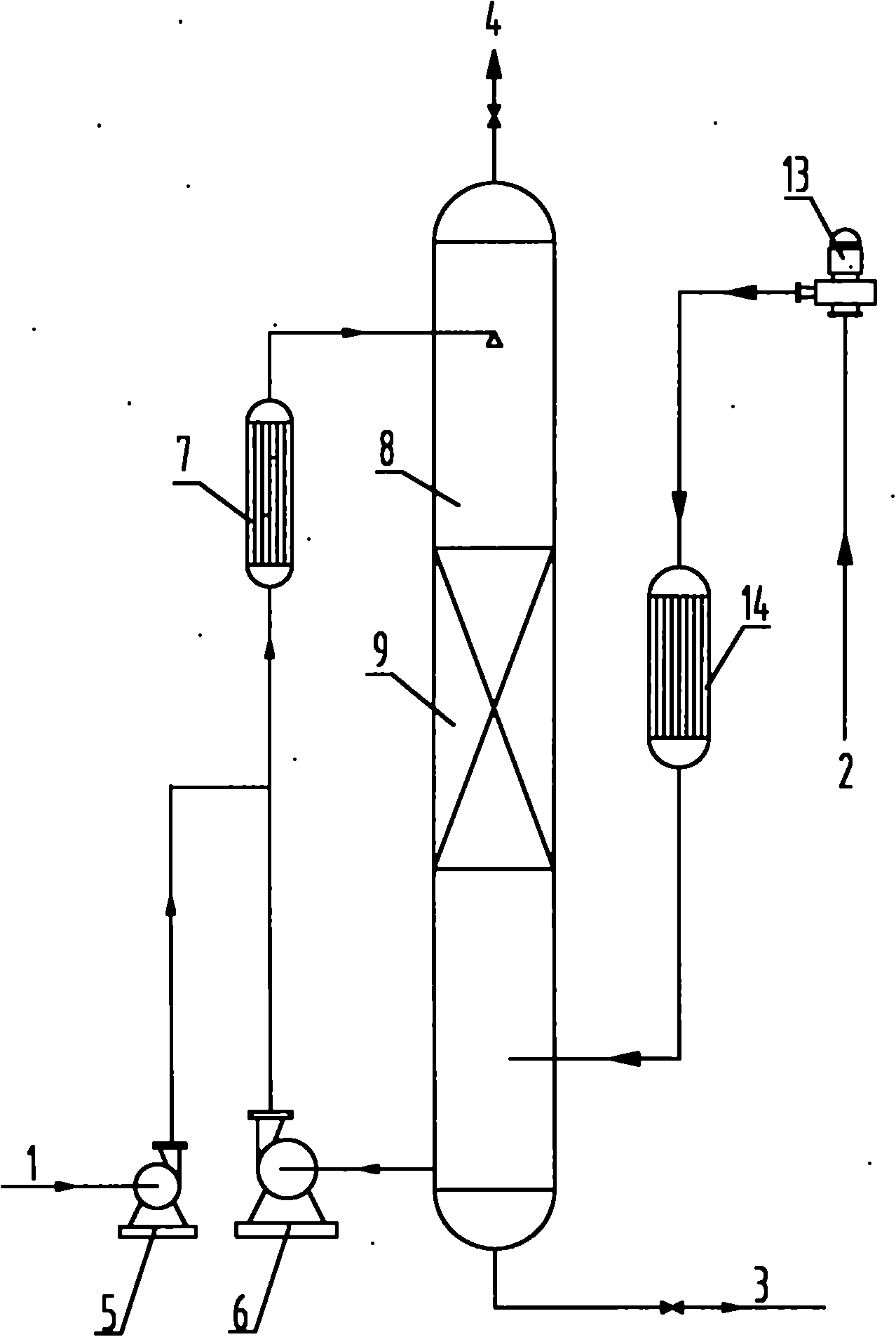
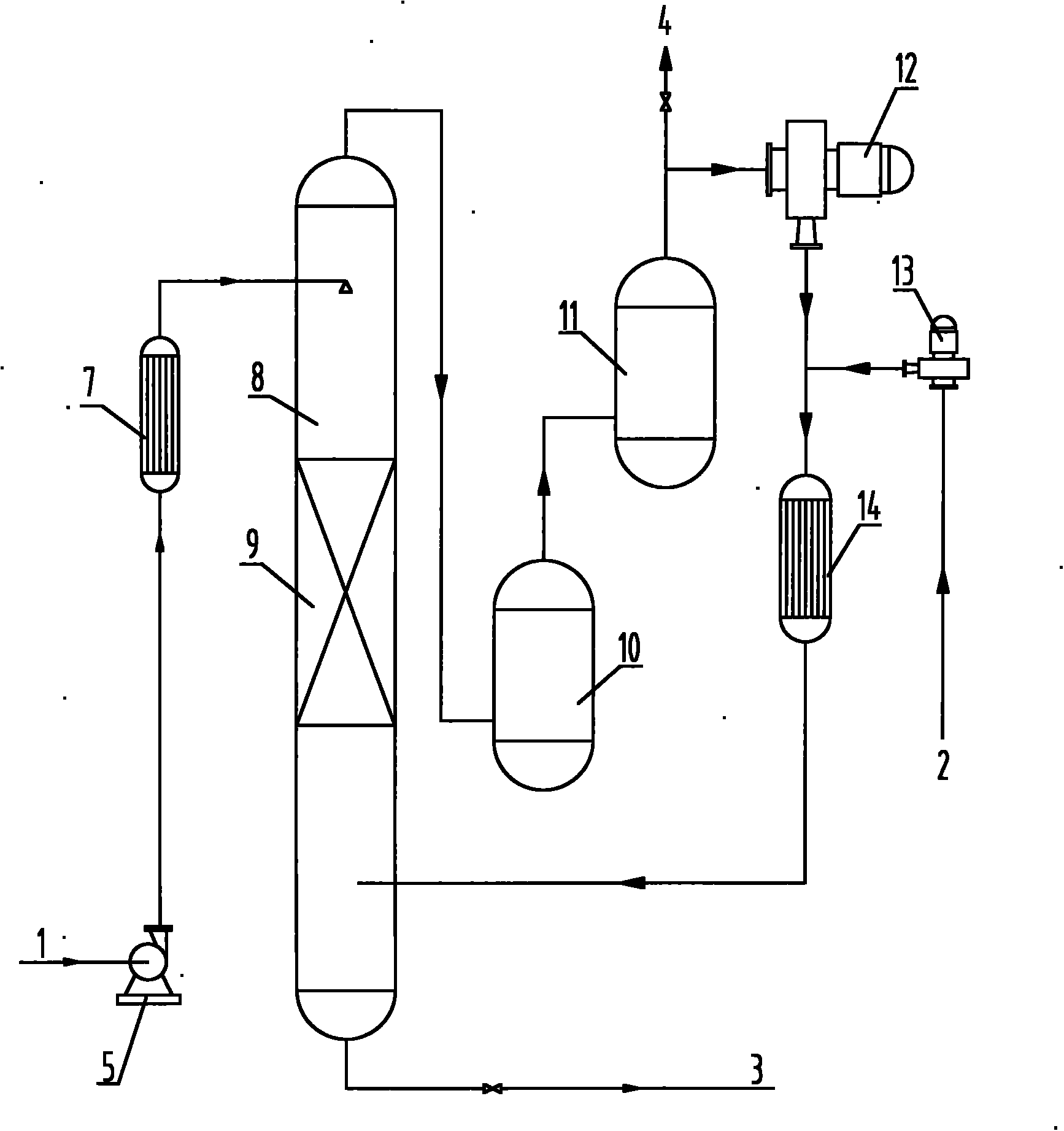
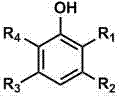
Preparation method of p-benzoquinone compound
The invention provides a preparation method of a p-benzoquinone compound. The preparation method is characterized in that the p-benzoquinone compound is prepared from a phenol compound with high selectivity under a mild condition by taking oxygen or oxygen-containing gas in a liquid solvent as an oxidizing agent in the presence of a transitional metal compound serving as a main catalyst and N-substituted hydrocarbon oxy-compound serving as an assistant catalyst.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Methods for oxidation of alpha tocotrienol in the presence of non-alpha tocotrienols
ActiveUS9162957B2Shorten the progressReduce or eliminate either a diseaseSenses disorderOrganic compound preparationStereoisomerismAlpha-Tocotrienol
A method of producing alpha-tocotrienol quinone or a stereoisomer thereof, the method comprising selective opening of alpha-tocotrienol chroman to alpha-tocotrienol quinone in the presence of non-alpha tocotrienol chromans by oxidizing alpha-tocotrienol with a metal salt oxidizing agent, wherein the stoichiometric ratio of metal salt oxidizing agent / alpha-tocotrienol is at least 4:1 and wherein said metal oxidizing agent is added in sequential additions, in order to reduce oxidation of any amounts of non-alpha tocotrienol chromans that might have been present in the starting alpha-tocotrienol chroman material. This process uses conditions favoring oxidation rates of the alpha tocotrienol chroman vs. the non-alpha tocotrienol chromans.
Owner:PTC THERAPEUTICS INC
Method for oxidizing phenol
ActiveCN102757301AHigh selectivityLow costOxygen-containing compound preparationMolecular sieve catalystsCatalytic oxidationTitanium
The invention provides a method for oxidizing phenol. The method comprises the following steps of: making phenol and hydrogen peroxide contact with a catalyst under an oxidizing reaction condition, and is characterized in that: the catalyst contains titanium silicalite and a multi-metal oxygen-containing acid and / or multi-metal oxysalt. The method has the advantages of adoption of an environment-friendly synthesizing process, environment friendliness, low cost, no need of adding any inhibitor or initiator, no requirement on special production equipment, simple production process, easiness for controlling and contribution to industrial production and application. Moreover, the method has high catalytic oxidation activity and high benzoquinone selectivity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for preparation of 2-Methyl-1,4-naphthoquinone
InactiveUS6579994B2Low costReduce usageOrganic compound preparationQuinone preparation by oxidationAcetic acidNaphthoquinone
The present invention describes a process for the preparation of 2-Methyl-1,4-naphthoquinone by oxidizing 2-methylnaphthalene with hydrogen peroxide in the presence of acetic acid.
Owner:COUNCIL OF SCI & IND RES
Method for directly oxidizing benzyl-position C-H bond into ketone
ActiveCN107011133AEfficient synthesisAtom economy is highCarboxylic acid nitrile preparationOrganic compound preparationSide chainEthyl group
The invention discloses a method for directly oxidizing a benzyl-position C-H bond into ketone, wherein aryl ethyl compounds are catalyzed and oxidized by nitrite ester; a synergistic catalytic system of free radical initiator and nitrite ester is adopted, and a catalytic system of non-metallic catalyst and oxygen is adopted, the oxidization of the C-H bond of a free radical-activated aryl side chain is simple in operation; after completing the reaction, petroleum ether / ethyl acetate at a volume ratio of (50-1):1 is used as an eluent; column chromatography separation is performed to obtain a target product. The catalytic system in the invention uses oxygen as an oxygen source and has high atomic economy; the invention is a non-metallic catalytic system and provides a novel method for avoid metal residues in synthetic drugs; for diethyl aromatic hydrocarbon, the method provided by the invention can be adopted to selectively oxidize diethyl aromatic hydrocarbon into monoketone and diketone; the method of the invention can be adopted to efficiently synthesize tranquillizer lenperone, so that a novel method for synthesizing lenperone is provided.
Owner:UNIV OF SCI & TECH OF CHINA
Method for direct oxidation of phenol compound to prepare p-benzoquinone compound
The invention provides a method for direct oxidation of a phenol compound to prepare a p-benzoquinone compound. The method is characterized in that: the phenol compound is taken as the raw material, oxygen or an oxygen-containing gas is used as an oxidant in a liquid medium, under the action of a transition metal compound main catalyst and an amphipathic molecule cocatalyst, and under mild conditions, highly selective preparation of the p-benzoquinone compound is carried out.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Process for synthesizing benzoquinones by direct oxidation of phenols
The invention discloses a process for synthesizing benzoquinone substances by direct oxidation of phenol substances. The synthesis process comprises the following steps of: dissolving the phenol substances serving as raw materials in a liquid solvent, and directly oxidizing the phenol substances into the corresponding benzoquinone substances by using oxygen-containing gas under the catalysis of a solid catalyst and / or a catalyst capable of being dissolved in a liquid medium. The process is characterized in that: because a reaction tower provided with an atomizing nozzle and / or a bulk copper wire filler is adopted, the liquid and the gas are fully contacted and uniformly mixed in the reaction tower, the gas and the liquid have as large inter-phase boundary as possible, the comprehensive rate of reaction is improved, side reaction is reduced, and the selectivity of the benzoquinone substances serving as target products is improved; and the whole process can be continuously operated, and can also be intermittently operated. The generated benzoquinone substances can be further reduced into benzenediols (catechol and / or hydroquinone) by using traditional reduction methods.
Owner:BEIJING BOYUAN HENGSHENG HIGH TECH
Method for preparing p-benzoquinone compound through selective catalytic oxidation of phenol compound
The invention provides a method for preparing a p-benzoquinone compound through selective catalytic oxidation of a phenol compound. The method is characterized in that the p-benzoquinone compound is highly selectively prepared through a reaction of a raw material the phenol compound in a liquid medium under mild conditions under the action of a transition metal compound primary catalyst and a stellate molecule cocatalyst with oxygen or an oxygen-containing gas as an oxidant.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Practical, cost-effective synthesis of ubiquinones
InactiveUS20050148675A1Convenient and efficient and inexpensive entryEfficient and inexpensiveBiocideOrganic compound preparationCombinatorial chemistryAlkyne
The present invention provides a convergent method for the synthesis of ubiquinones and ubiquinone analogues. Also provided are precursors of ubiquinones and their analogues that are useful in the methods of the invention. The invention further provides an improved method for the carboalumination of alkyne substrates.
Owner:ZYMES
Method for synthesizing 2-methyl-1, 4-naphthoquinone
InactiveCN102516054AAvoid pollutionMild conditionsQuinone preparation by oxidationIce waterPtru catalyst
The invention relates to a method for synthesizing 2-methyl-1, 4-naphthoquinone through taking 2-methylnaphthalene as a raw material, which comprises the following steps of: taking the 2-methylnaphthalene as the raw material, taking glacial acetic acid as a solvent, taking hydrogen peroxide as an oxidizing agent and catalyzing with a catalyst to synthesize the 2-methyl-1, 4-naphthoquinone. The reaction temperature is 20-80 DEG C, and the dripping rate is 0.02-1 mL / min. After the end of dripping, the temperature is preserved for 0.5-4 hours. After the reaction is ended, and feed liquid is concentrated to 1 / 2-1 / 6 of the original volume, the feed liquid is poured into ice water with the volume being 3-6 times that of the feed liquid and is then stewed, filtered and dried to obtain the 2-methyl-1, 4-naphthoquinone. The method has the beneficial effects that a mixed acid catalytic system is used; compared with a traditional chromate oxidation method, the method has the advantages of good selectivity and high yield; and the pollution to a large quantity of chromium metal ions generated in the traditional industrial production can be avoided, and therefore, the industrial application value is higher.
Owner:SOUTHEAST UNIV
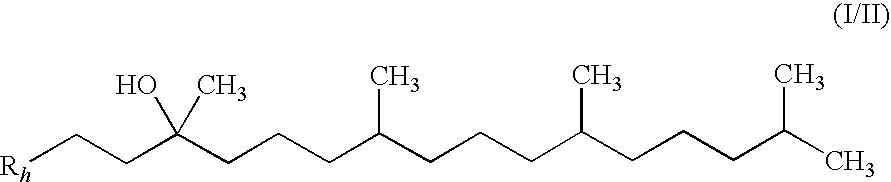
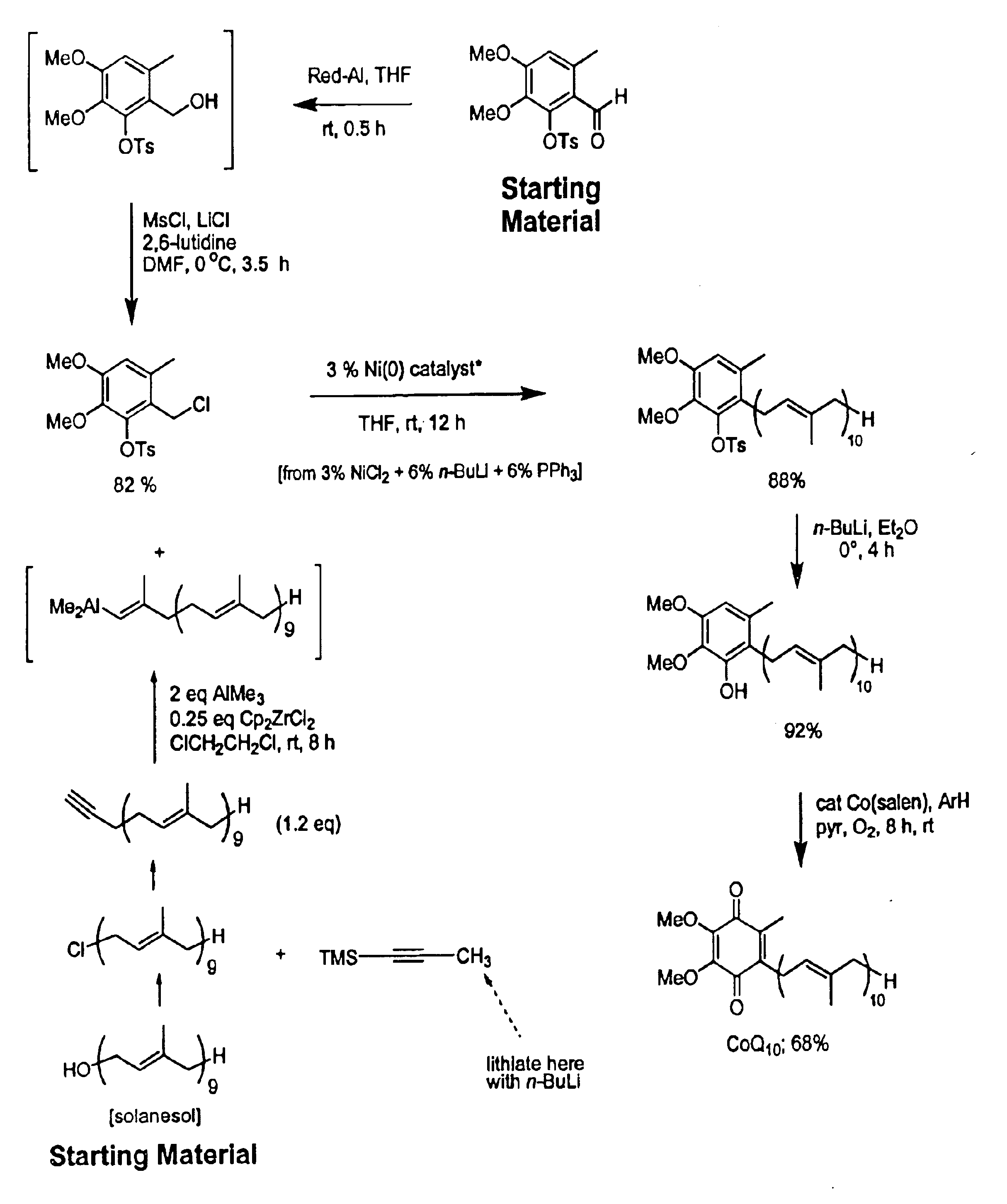
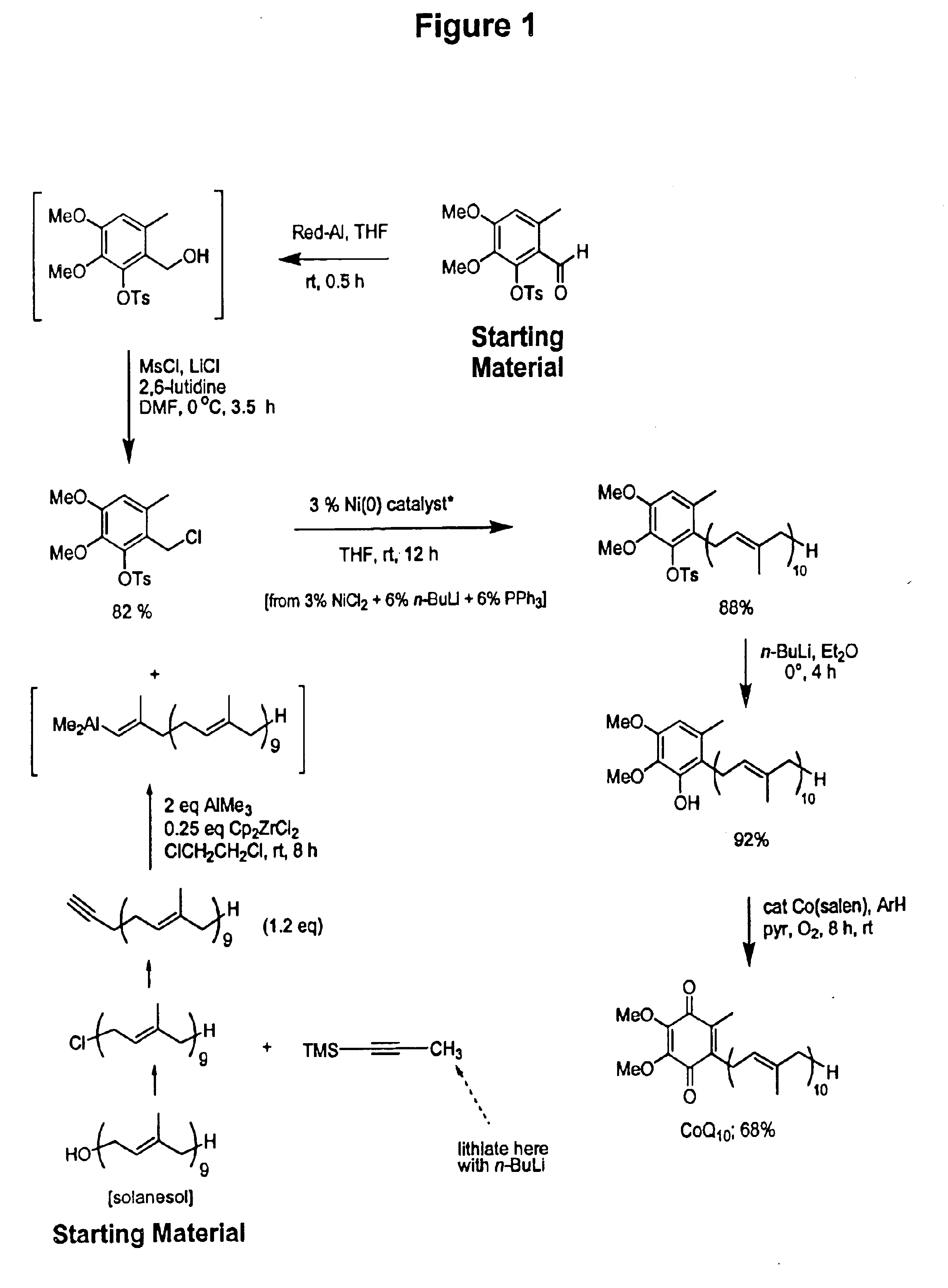
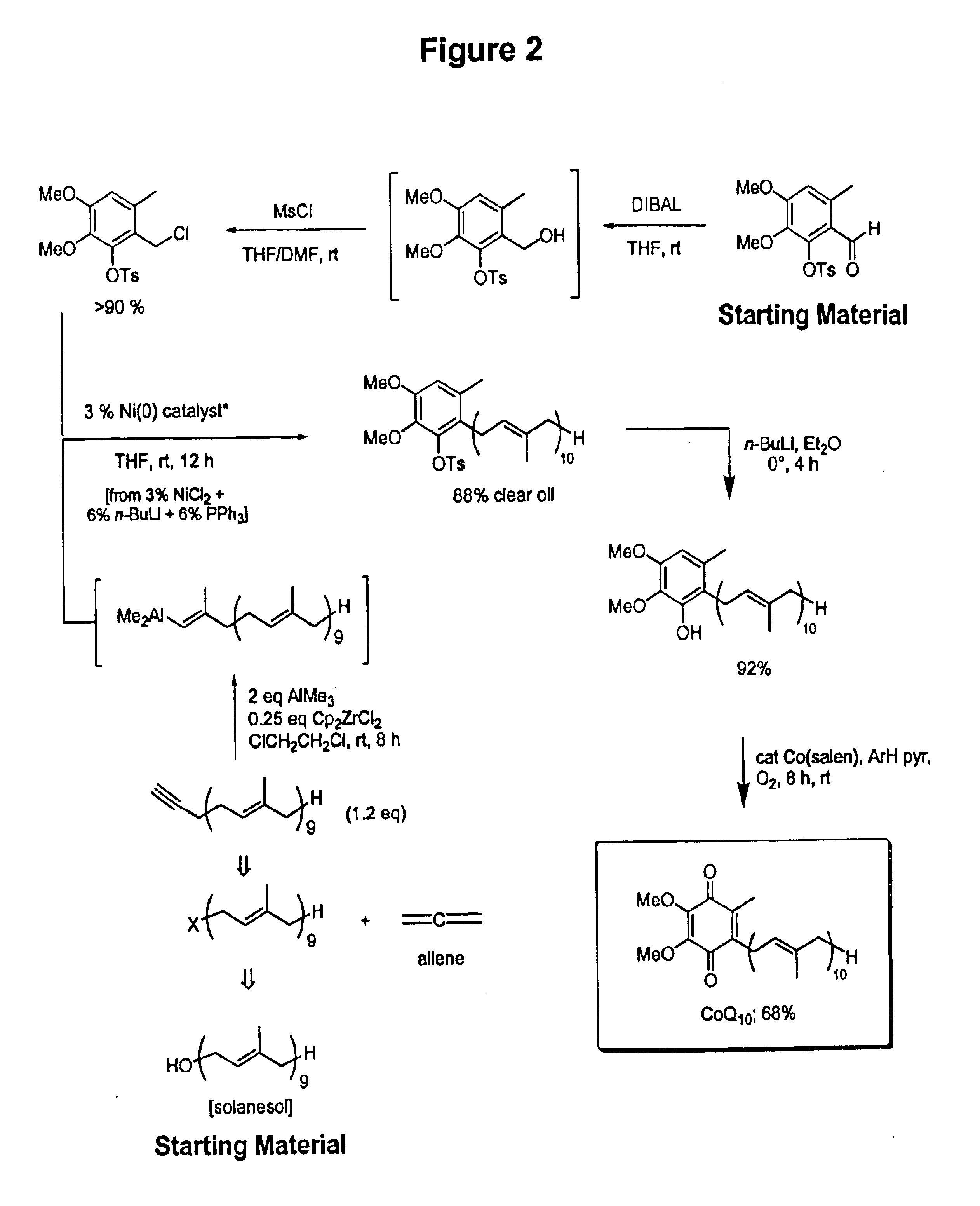
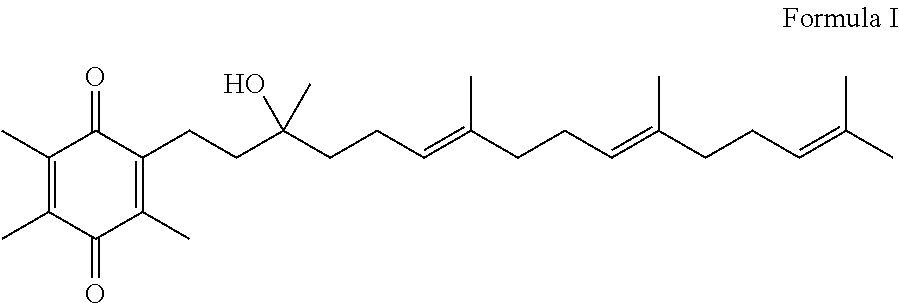
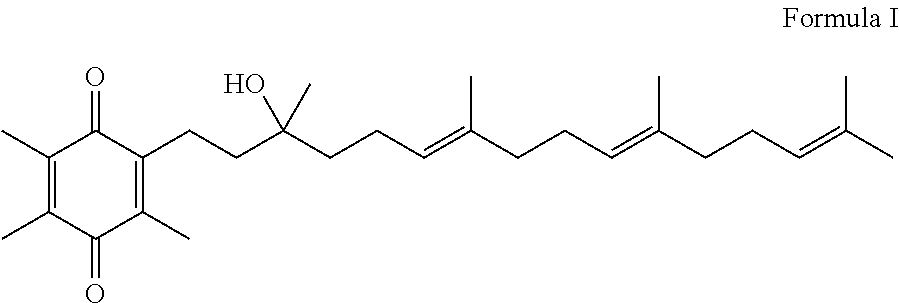
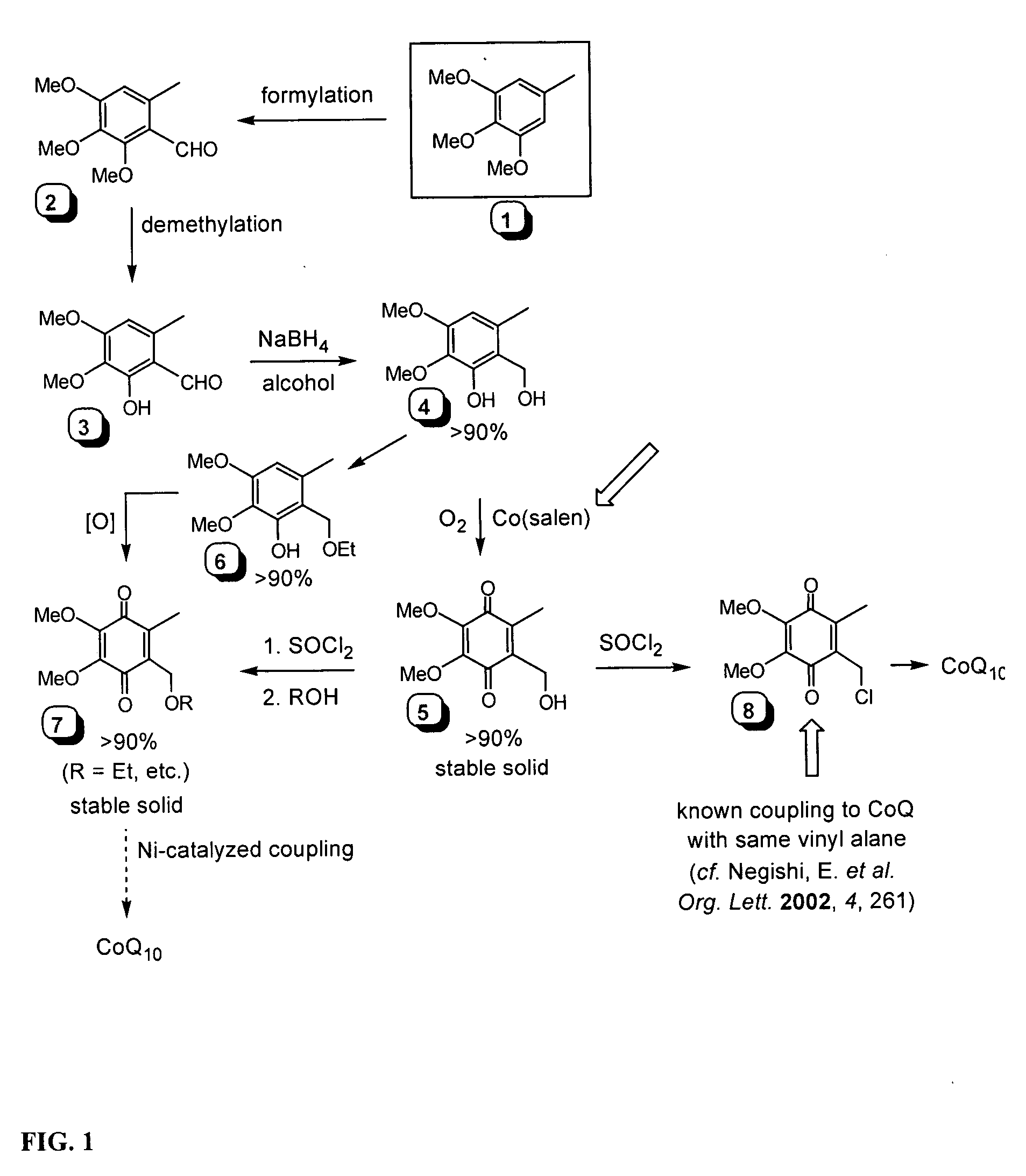
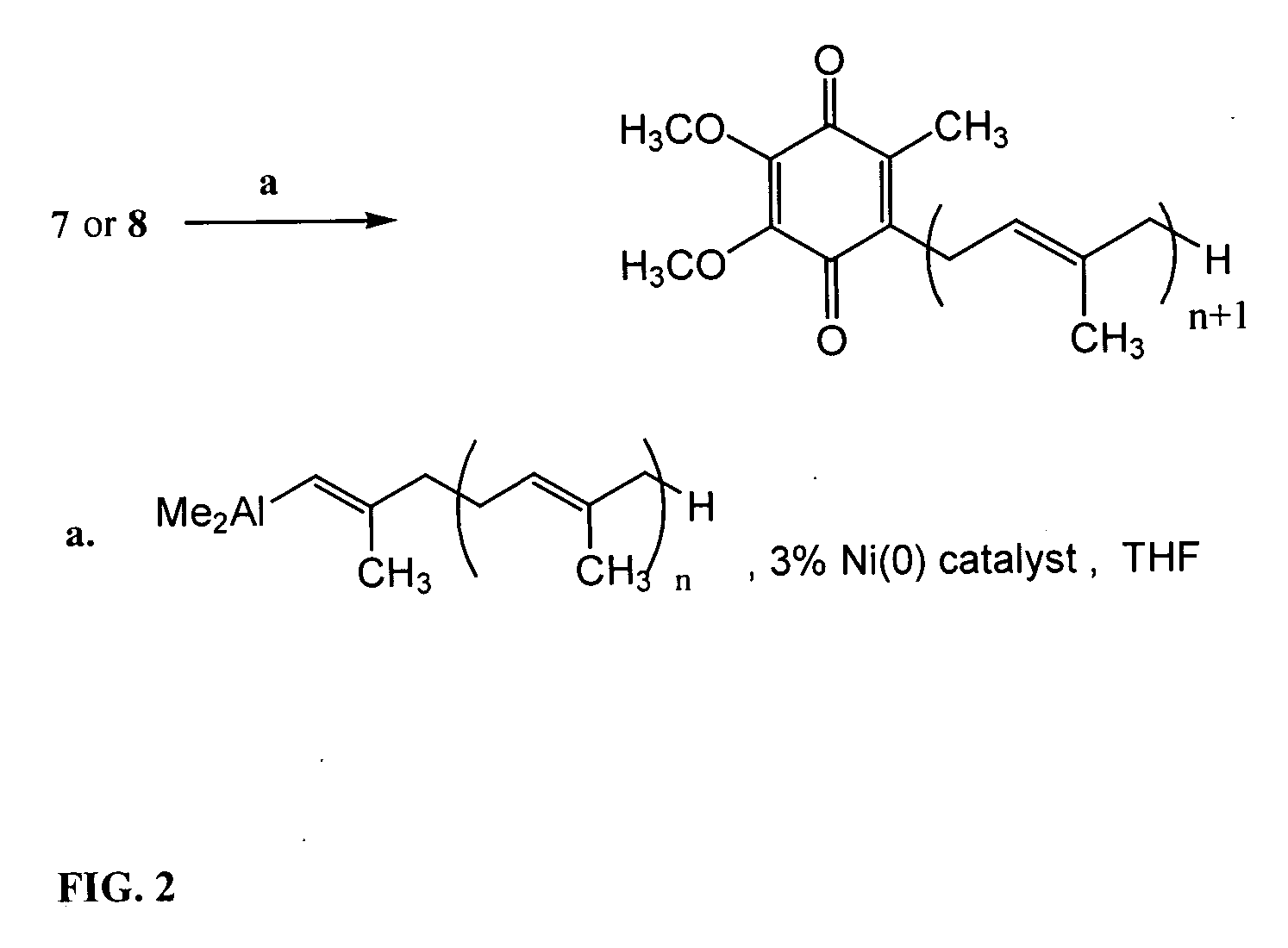
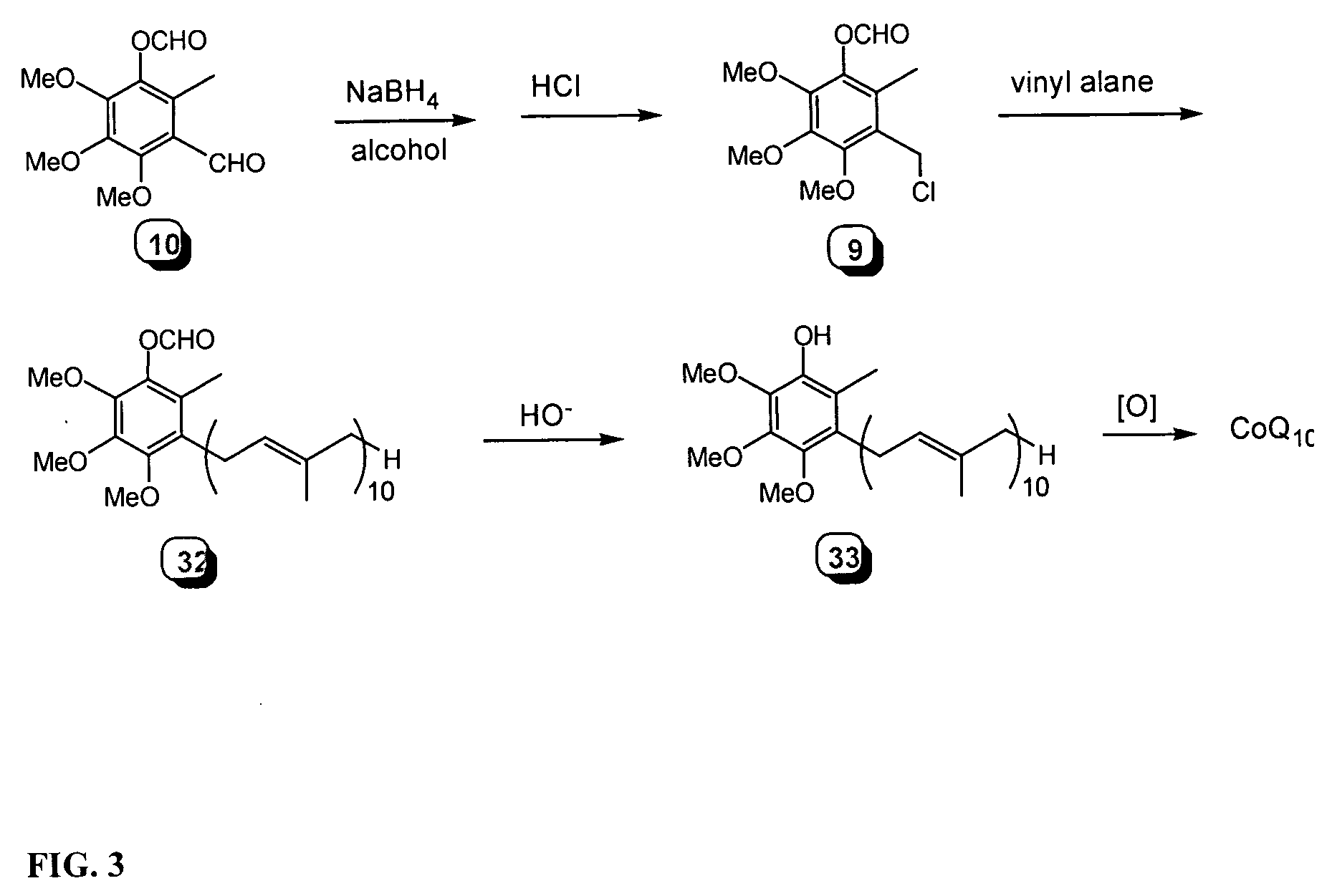
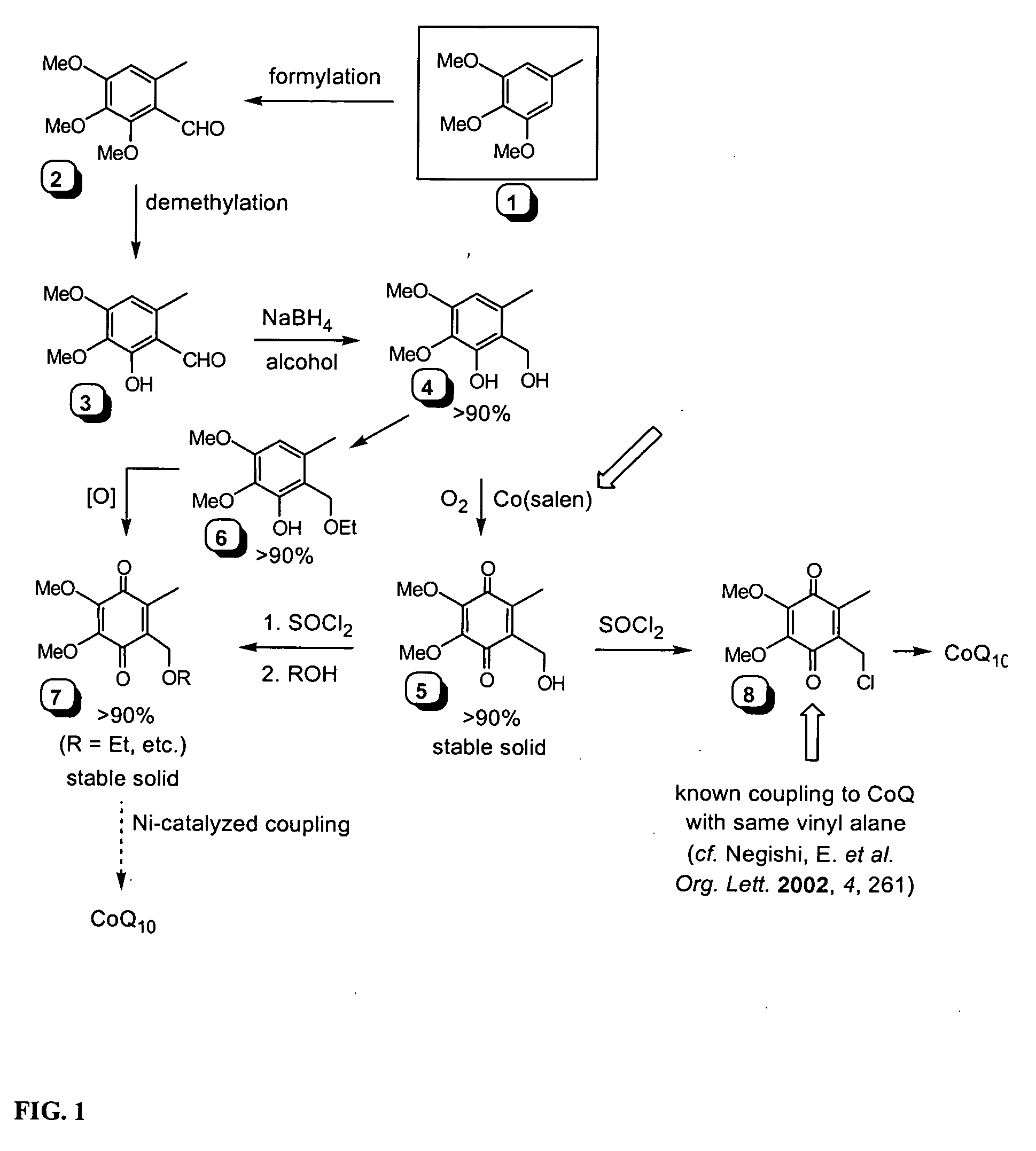
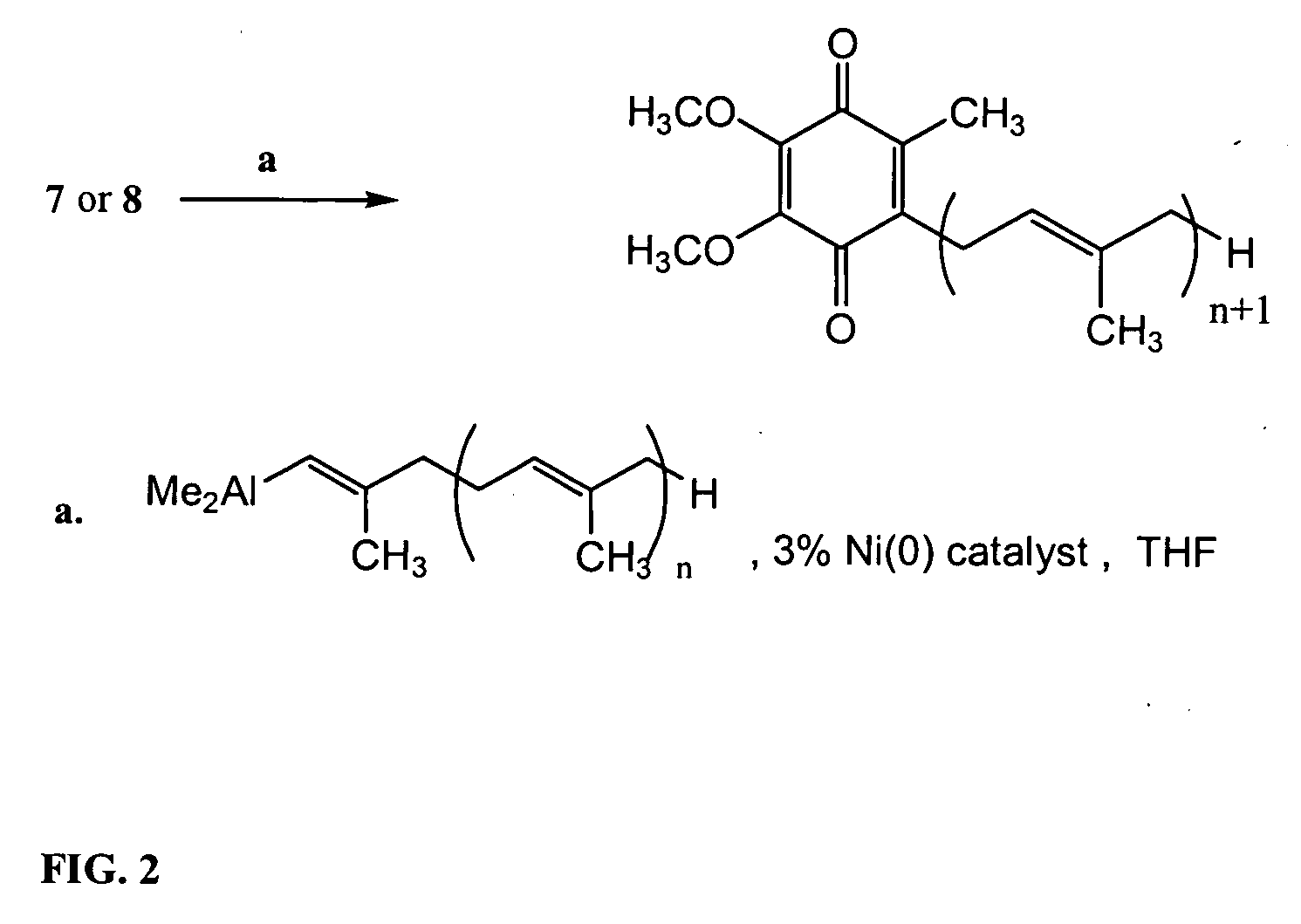
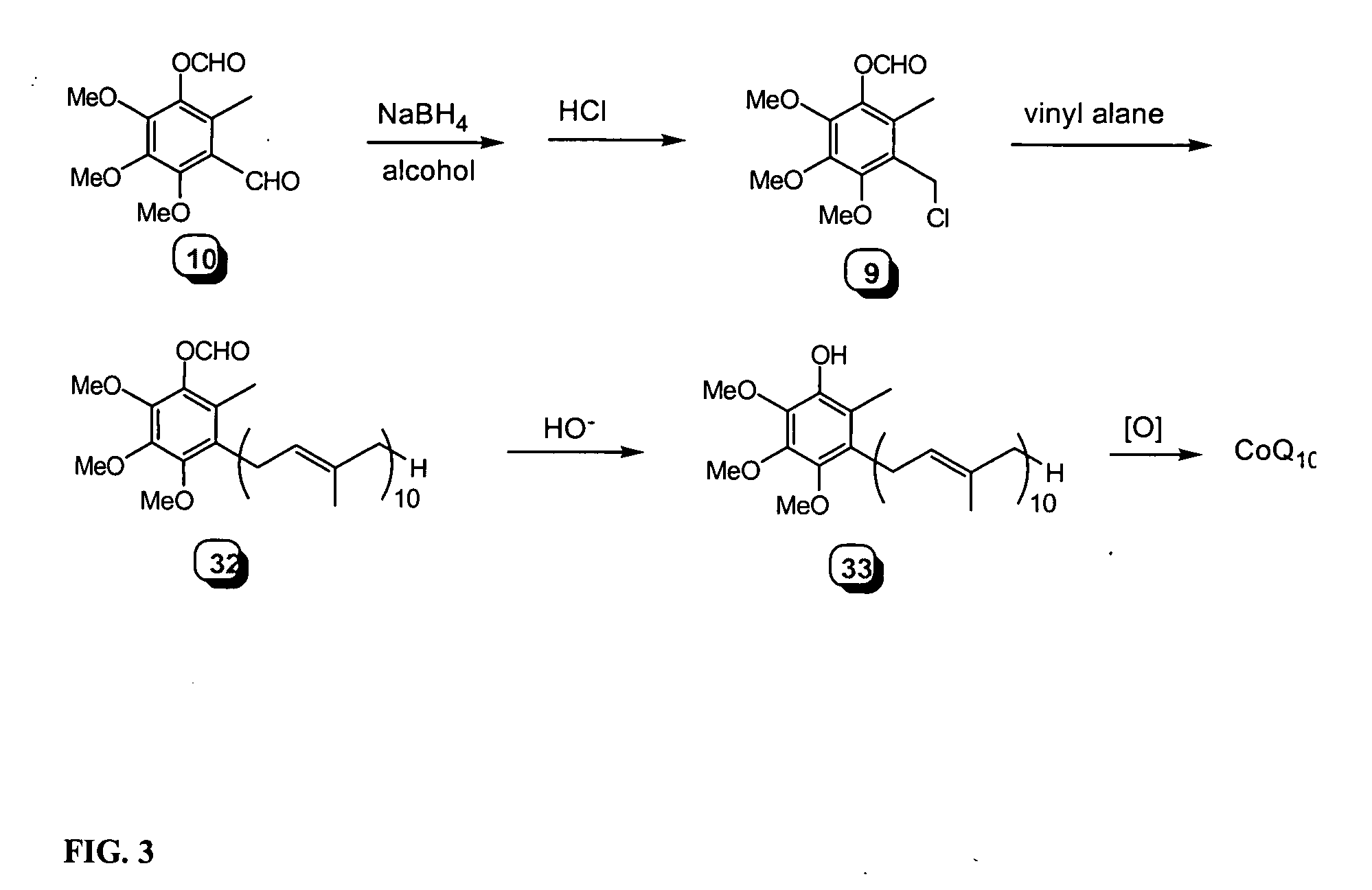
Novel Intermediates, Process for Their Preparation and Process for the Preparation of Coq10 Employing the Said Novel Intermediates
InactiveUS20080200702A1Speed up the processSimple and cost-effective and commercially applicableOrganic compound preparationQuinone preparation by oxidation1,4-BenzoquinoneMethyl group
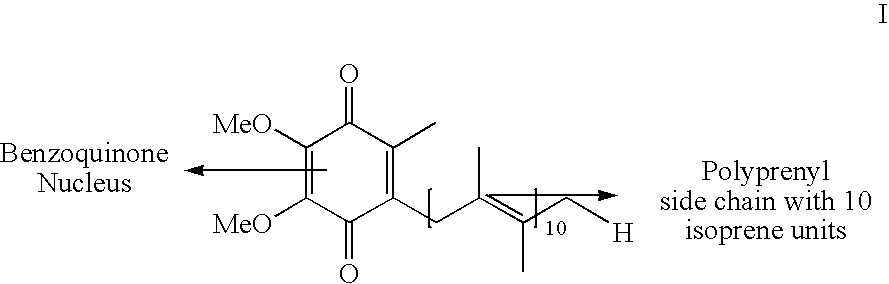
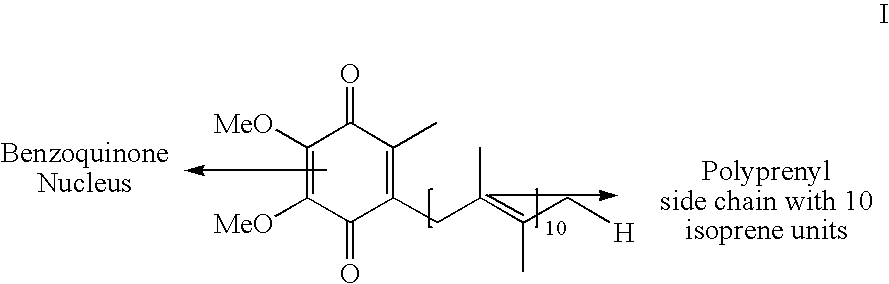
The present invention relates to an improved process for the preparation of Coenzyme Q. Coenzyme Q10 or CoQ10 has the chemical name 2-[(all-trans)-3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl]-5,6-dimethoxy-3-methyl-1,4-benzoquinone and has the formula I.The invention also provides new intermediates useful for the preparation of CoQ10 and processes for their preparation.
Owner:NICHOLAS PIRAMAL INDIA LTD
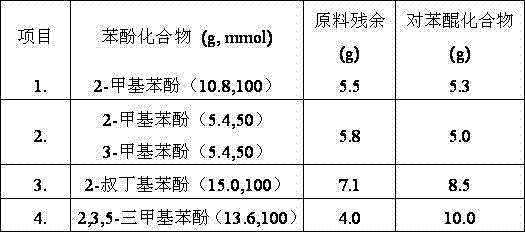
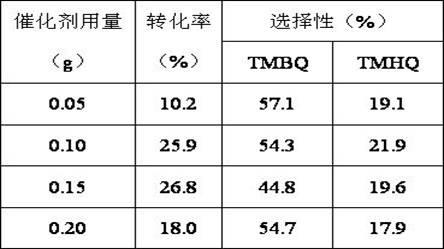
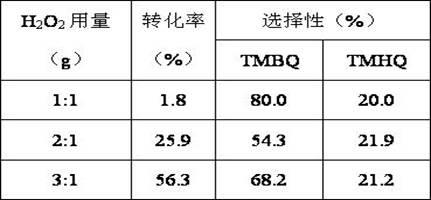
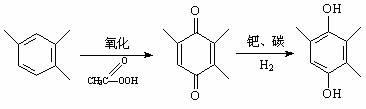
Method for synthesizing 2,3,5-trimethylbenzoquinone and 2,3,5-trimethylhydroquinone
InactiveCN102219665AFacilitated DiffusionEasy to separateOrganic compound preparationQuinone preparation by oxidationMolecular sieveAcetonitrile
The invention provides a method for synthesizing 2,3,5-trimethylbenzoquinone and 2,3,5-trimethylhydroquinone. In the method, the 2,3,5-trimethylbenzoquinone and the 2,3,5-trimethylhydroquinone are prepared by oxidizing reaction at the temperature of between 50 and 80 DEG C in the presence of a Cu-SBA-15 mesoporous molecular sieve serving as a catalyst, H2O2 serving as an oxidant and acetonitrile serving as a solvent. In the method, the reaction process is simple, and the equipment requirement is low; the Cu-SBA-15 mesoporous molecular sieve is used as the catalyst, contributes to the dispersion of products and is easy to separate; and the H2O2 is used as the oxidant, and the byproduct is H2O, so that the method is environment-friendly.
Owner:YANGZHOU UNIV
Method for preparing anthraquinone through anthracene oxidation
ActiveCN106966884AHigh selectivityHigh yieldQuinone preparation by oxidationHeterogenous catalyst chemical elementsAnthraceneMetal
The invention discloses a method for preparing anthraquinone through anthracene oxidation. The method includes: using oxide of aluminum as a carrier and metal oxide as an active center of a catalyst to catalyze anthracene oxidation to prepare anthraquinone, and specifically includes: 1, preparing a metal oxide compound catalyst; 2, allowing reaction to prepare anthraquinone. The metal oxide compound catalyst used in the method belongs to environment-friendly solid catalysts, specific surface area and pore size of the metal oxide compound catalyst can be adjusted by controlling temperature to control reaction, and the catalyst is easy to separate after reaction for preparing anthraquinone through anthracene oxidation is finished. The process of preparing anthraquinone through anthracene oxidation is simple to operate, the metal oxide compound catalyst is high in activity, recyclable and free of generating waste, and the method is environment-friendly.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for the preparation of 2,3,5-trimethyl-p-benzoquinone
InactiveUS6262311B1Highly active andSolution to short lifePhysical/chemical process catalystsOrganic compound preparationRare earthOxygen
Process for the preparation of 2,3,5-trimethyl-p-benzoquinone by oxidizing 2,3,5-trimethylphenol or 2,3,6-trimethylphenol with oxygen or an oxygen-containing gas mixture in the presence of a catalyst system which can be a copper halide and a transition metal halide; for example iron, chromium, manganese, cobalt, nickel, zinc or a rare earth halide, in a two-phase reaction medium, at elevated temperature.
Owner:DEGUSSA AG
Method for preparing 2,3,5-trimethylhydroquinone
The invention discloses a method for preparing 2,3,5-trimethylhydroquinone, which comprises oxidizing 2,3,6-trimethylphenol with pure oxygen or oxygen-enriched gas in water serving as a reaction medium in the presence of a catalyst consisting of copper halide and lithium halide and a phase transfer catalyst to obtain 2,3,5-trimethylhydroquinone. In the method disclosed by the invention, organic solvent is not added, the production safety is ensured, the operation is simplified, the energy cost is greatly reduced, the yield of the obtained product is over 90 percent, and the purity of the obtained product is over 99.5 percent. Thus, the method is suitable for industrial mass production.
Owner:SHANGHAI HEGNO PHARMA HLDG +1
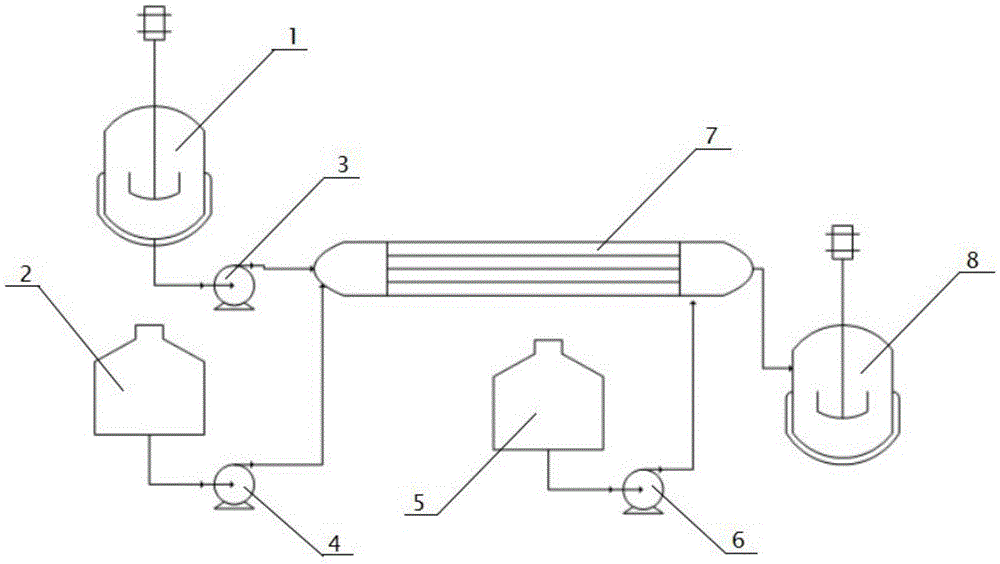
Tubular continuous method for preparing beta-menadione
ActiveCN105481673AImprove production continuityHigh yieldQuinone preparation by oxidationAutomatic controlRetention time
The invention discloses a tubular continuous method for preparing beta-menadione, which comprises the following steps: preparing a beta-methylnaphthalene emulsion, heating the beta-methylnaphthalene emulsion, metering the beta-methylnaphthalene emulsion and an oxidation solution respectively by a measuring pump, adding into a tubular reactor provided with an ultrasonic generator, and carrying out ultrasonic reaction, wherein the retention time of the reactants in the tubular reactor is 1-300 minutes; supplementing dilute sulfuric acid to the outlet of the tubular reactor, adding into a curing tank, and curing for 1-10 hours while keeping the temperature; after finishing curing, filtering, washing, carrying out centrifugal dehydration and drying to obtain the beta-menadione finished product. By using the tubular reactor to produce the beta-menadione, the method has the advantages of high production continuity and short period; and the product has the advantages of high yield, low impurity content and stable quality. The reaction process is easy to control, simple to operate and high in automatic control degree; the material performs forced flow in the tubular reactor to substitute mechanical stirring, thereby implementing industrialized continuous production; and thus, the method has the characteristics of low input cost and short reconstruction period, and can perform large-scale industrial production.
Owner:SICHUAN YINHE CHEM
Practical, cost-effective synthesis of ubiquinones
InactiveUS20060167289A1Efficient and inexpensive methodConvenient and efficient and inexpensive entryGroup 4/14 element organic compoundsOrganic compound preparationAlkyneCombinatorial chemistry
The present invention provides a convergent method for the synthesis of ubiquinones and ubiquinone analogues. Also provided are precursors of ubiquinones and their analogues that are useful in the methods of the invention. The invention further provides an improved method for the carboalumination of alkyne substrates.
Owner:ZYMES
Total synthesis method for preparing receme alkannin
InactiveCN1686994ARaw materials are easy to getFew synthetic stepsQuinone preparation by oxidationBulk chemical productionSide chainSolvent
The present invention relates to a total synthesis method for preparing racemate alkannin. Said invention uses naphthaldehyde protected by protecting group as raw material and adopts the following steps: heating said raw material and metal oxide of 1-halogeno-3-methyl-2-butylene and making then produce reaction in aprotic polar solvent, zone-selectively making alpha-addition reaction, and introducing side chain of alkannni to obtain 2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxy- aphthalene or 2-(1-hydroxy-4-methyl-3-pentenyl)-1,8:4,5-bis( methylene-dioxy) naphthalene, then making oxidation to remove protecting group so as to obtain racemic alkannin.
Owner:SHANGHAI JIAO TONG UNIV
Novel synthesis method of 2-methyl-1,4-naphthoquinone
InactiveCN103833541AReduce consumptionEasy to separateQuinone preparation by oxidationSynthesis methodsDistillation
The invention relates to a novel synthesis method of 2-methyl-1,4-naphthoquinone. The novel synthesis method comprises the steps: respectively dissolving a defined amount of 2-methylnaphthalene and metachloroperbenzoic acid in moderate glacial acetic acid to prepare solutions (1) and (2), dropwise adding the solution (2) in the solution (1) under the stirring, after reacting for a period of time at a certain temperature, extracting a reaction solution by using chloroform, washing an extraction solution with a saturated NaHCO3 solution and water, after anhydrous Na2SO4 is dried, performing reduced pressure distillation to prepare a crude product of the 2-methyl-1,4-naphthoquinone, and recrystalizing by using alcohol as a solvent to obtain a pure product of the 2-methyl-1,4-naphthoquinone. According to the novel synthesis method, the conversion rate of the 2-methylnaphthalene can reach 80 percent, and the yield of the 2-methyl-1,4-naphthoquinone can reach 31 percent. The method disclosed by the invention has the characteristics of short reaction time, mild conditions, and the like, and products are easily separated; and no any catalyst is used in the reaction, thus the energy consumption and the environment pollution can be reduced.
Owner:QILU UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com