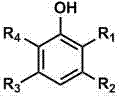
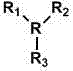Method for direct oxidation of phenol compound to prepare p-benzoquinone compound
A technology of phenol compounds and compounds, applied in the field of direct oxidation of phenol compounds to prepare p-benzoquinone compounds, which can solve the problem of excessive catalyst consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add manganese nitrate (2.5g, 10.0mmol), p-aminobenzoic acid (1.37g, 10mmol), phenol (9.4g, 100mmol) and acetonitrile (78.5g, 100mL) into a 250mL reactor, mix well, and close the reactor and filled with 30atm oxygen, stirred and heated to 60 o C, after reacting for 3 hours, cool to room temperature, take out the mixture for analysis after degassing, and obtain remaining phenol: 6.4g, p-benzoquinone: 2.9g.
[0033]
Embodiment 2-9
[0035] Manganese nitrate in embodiment 1 is replaced with different transition metal compounds, and the impact on reaction result is shown in the following table:
[0036] project Main catalyst (g, mmol) Phenol (g) p-Benzoquinone (g) 1. -- 9.2 0 2. Vanadium pentoxide (1.82, 10) 8.8 0.5 3. Copper Chloride Dihydrate (1.7, 10) 7.4 1.8 4. Iron Phthalocyanine (5.68, 10) 4.8 4.4 5. Cobalt nitrate hexahydrate (2.91, 10) 6.4 2.7 6. Ruthenium trichloride (2.1, 10) 7.1 2.2 7. Nickel acetate (1.76, 10) 4.1 4.8 8. Palladium chloride (1.77, 10) 6.1 3.2
Embodiment 10-18
[0038] The p-aminobenzoic acid in Example 1 is replaced with different amphiphilic compounds, and the impact on the reaction result is shown in the following table:
[0039] project Cocatalyst (g, mmol) Phenol (g) p-Benzoquinone (g) 1. -- 8.9 0.4 2. Ethanolamine (0.61, 10) 8.2 1.1 3. 2-Aminoacetic acid (0.75, 10) 6.0 3.1 4. 2-Aminophenylacetic acid (1.51, 10) 8.3 0.9 5. p-toluenesulfonic acid (1.72, 10) 7.1 2.1 6. 4,4'-biphenol (3.7, 10) 7.6 1.7 7. Ethyl 2-aminoacetate (1.03, 10) 5.9 3.1 8. Ethyl N,N-dimethylaminoacetate (1.32, 10) 8.6 0.6 9. Sodium octyl sulfonate (2.34, 10) 8.6 0.7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com