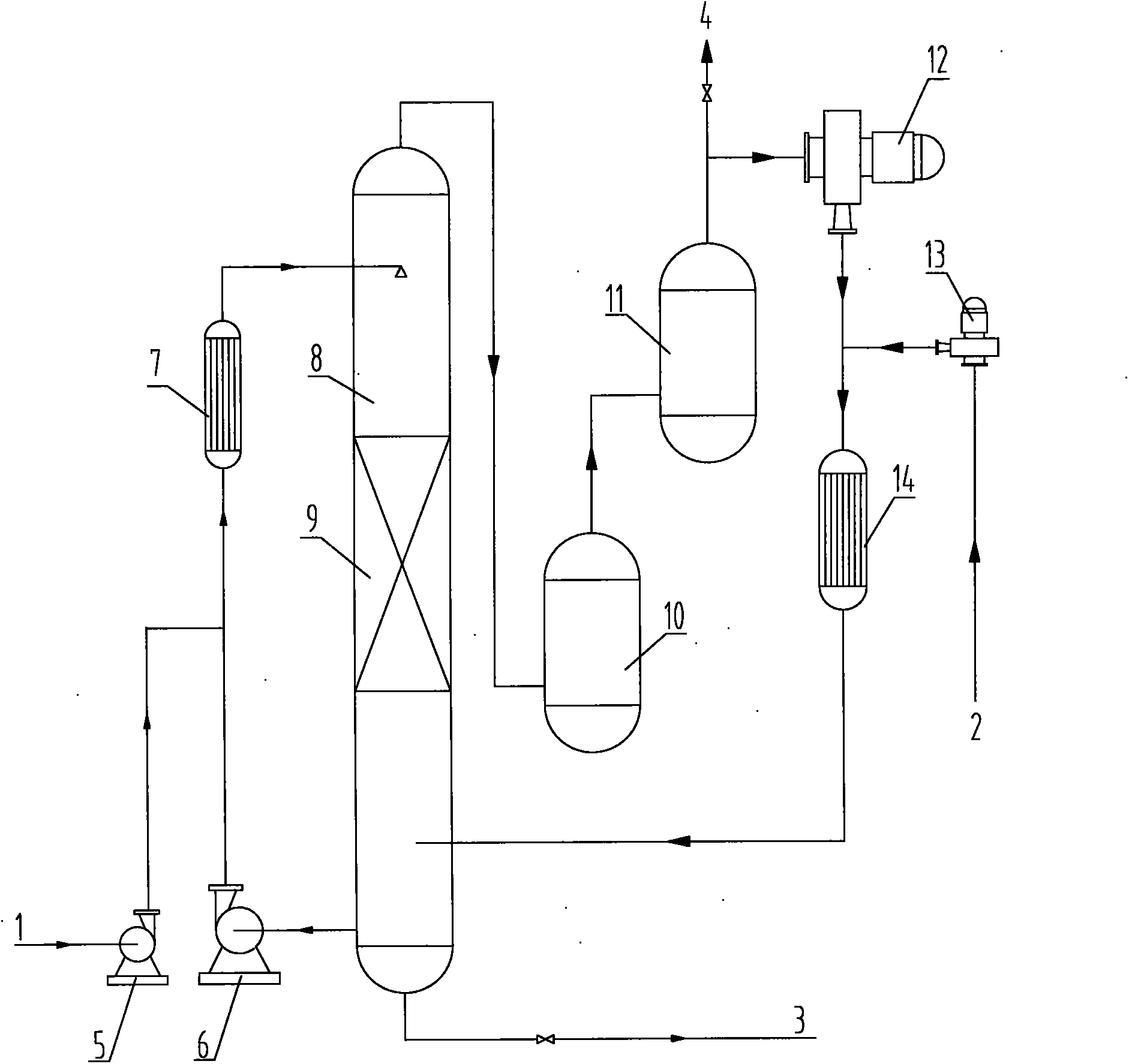
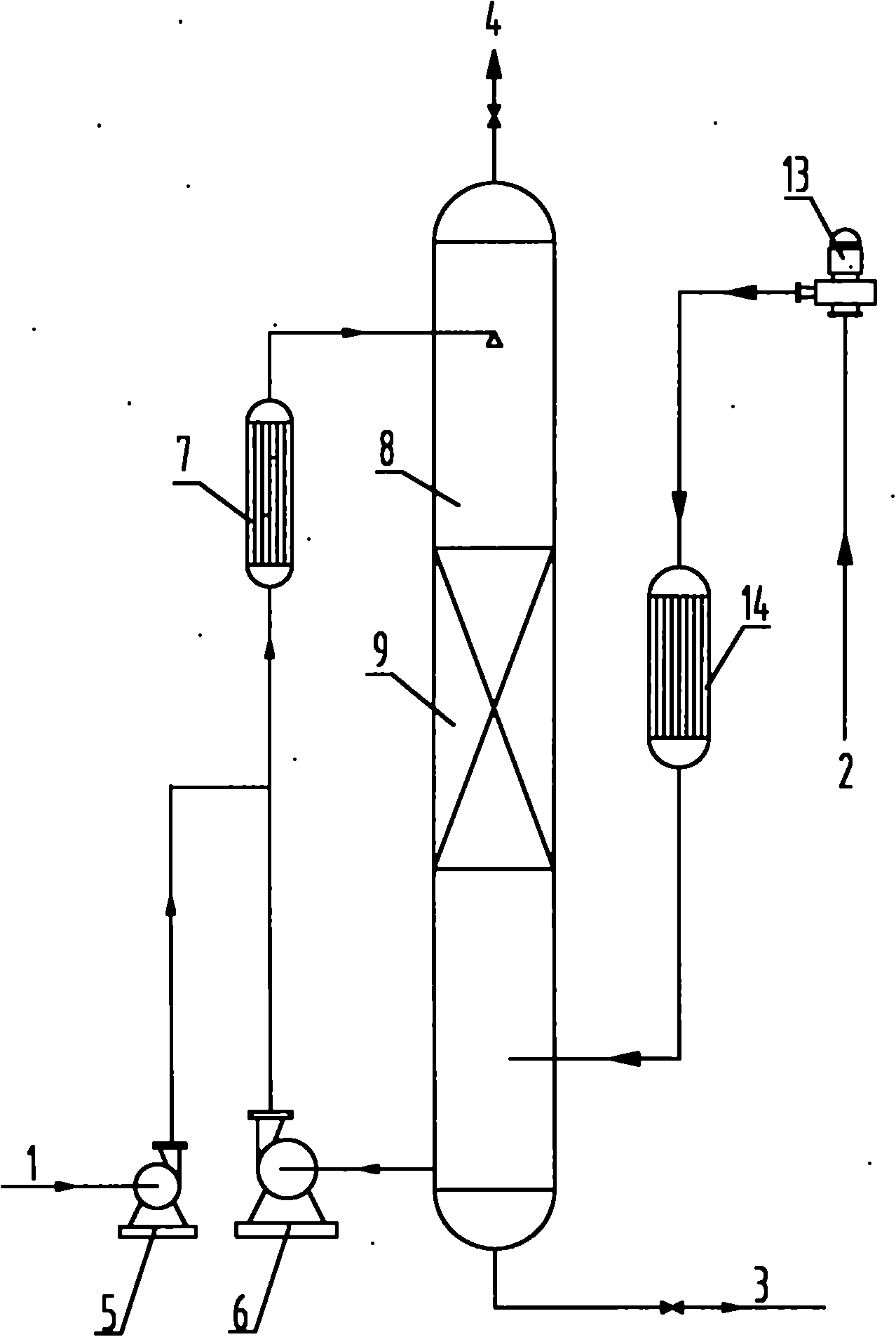
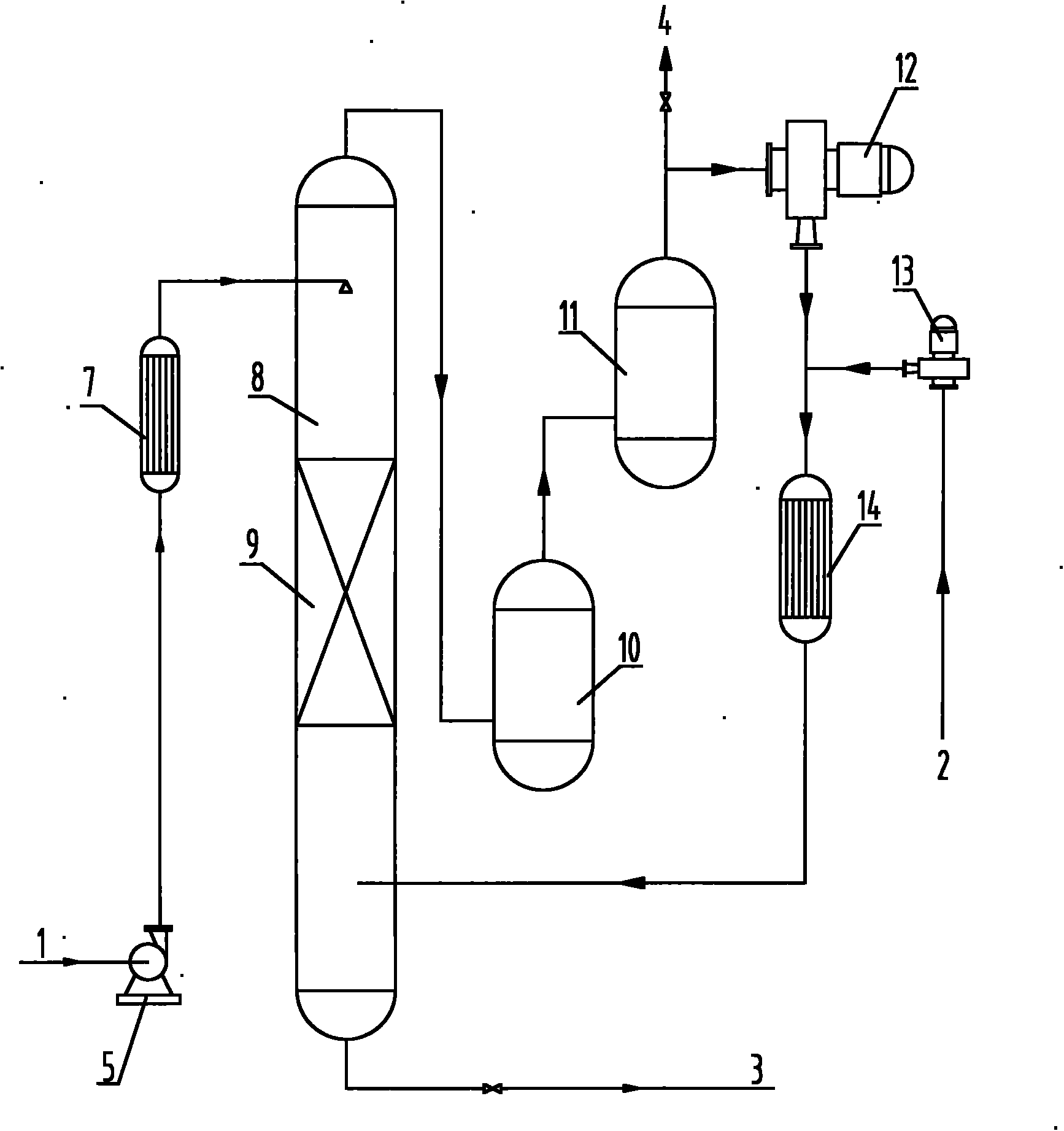
Process for synthesizing benzoquinones by direct oxidation of phenols
A technology of phenols and processes, applied in the preparation of oxidized quinones, organic chemistry, etc., can solve problems such as tar formation and target product yield reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] In distilled water, add 5% phenol (weight ratio based on liquid phase feed), and 2.5% CuCl 2 / NaCl (molar ratio)=1: 3 mixture (weight ratio based on liquid-phase feed) as catalyzer, control reaction temperature is 70-80 ℃, and reaction pressure is 3.0-3.2MPa, respectively in reactor (below Referred to as No. 1 reactor for short), traditional reaction tower (filler and large grain copper are housed in the reaction tower, particle diameter 3-10mm) (hereinafter referred to as No. 2 reactor), reaction tower of the present invention (with copper wire as filler , the diameter of the copper wire≤2mm) (hereinafter referred to as No. 3 reactor), the reaction tower (atomized feed) of the present invention (hereinafter referred to as No. 4 reactor), the reaction tower of the present invention (with copper wire as filler , simultaneously in the reactor of atomized feed form) (hereinafter referred to as No. 5 reactor), carry out oxidation reaction with oxygen as oxygenant, and react...
Embodiment 2
[0104] The distilled water among the embodiment 1 is replaced with methyl alcohol, other conditions are constant, and reaction result is as shown in the table below:
[0105]
Embodiment 3
[0107] The distilled water among the embodiment 1 is replaced with ethanol, other conditions are constant, and reaction result is as shown in the table below:
[0108]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com