Method for preparing 2-amylanthraquinone by two-step method
A technology for amyl anthraquinone and amyl anthracene, which is applied in the field of preparing 2-amyl anthraquinone by a two-step method, can solve the problems of excessive hydrogenation, hidden dangers in production safety, large equipment corrosion, etc., and achieves mild reaction conditions and reduced production. cost, the effect of reducing production pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
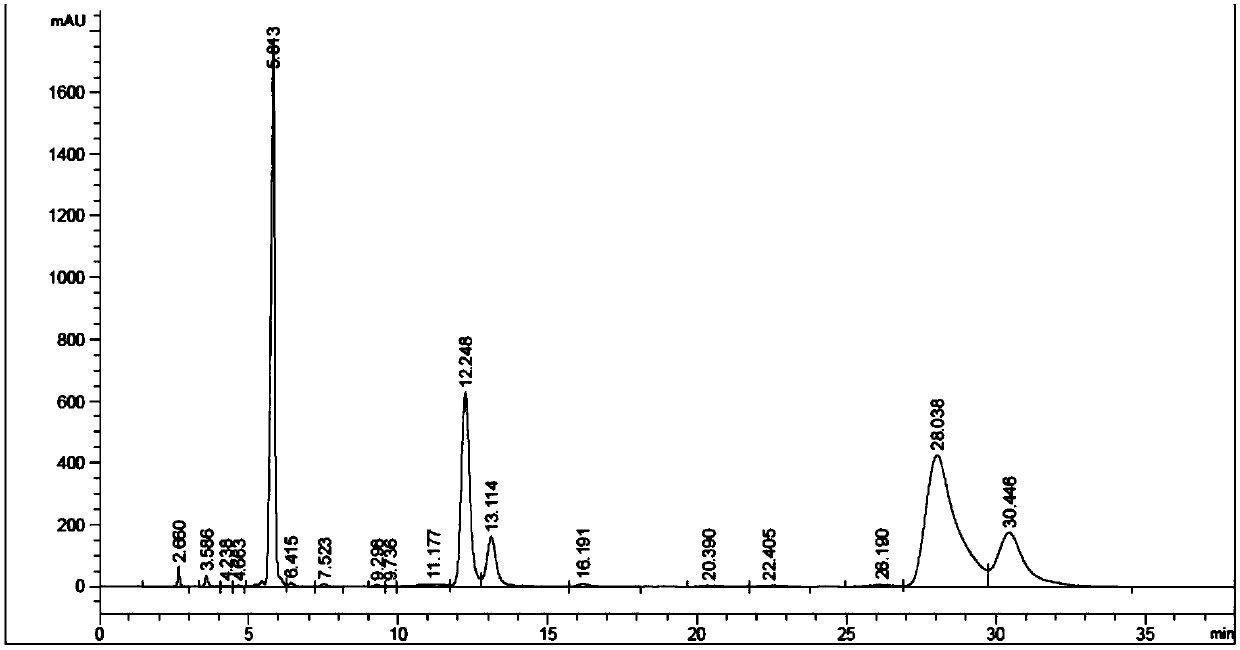
[0034] Step 1: In the autoclave, add 100g of anthracene, 50g of isopentene and 0.5g of Mg-MWW molecular sieve, and then add 150ml of solvent. After the autoclave is sealed, replace the air in the autoclave with nitrogen, and then heat the autoclave. At a reaction temperature of 200°C, fill the reactor with nitrogen to a reaction pressure of 2.5Mpa, and stir the reaction at the reaction temperature and pressure. During the reaction, continuously supply nitrogen and maintain a stable reaction pressure; Separation process: After the alkylation reaction is finished, take the reaction kettle out of the heating furnace and cool it to room temperature, reduce the pressure in the reaction kettle to normal pressure, open the lid of the kettle, take out the mixed solution, and carry out vacuum distillation on the mixed solution. Separation of catalyst and product 2-pentyl anthracene and unreacted raw material anthracene; obtain 2-pentyl anthracene;
[0035] The second step: mix the 2-amyl...
Embodiment 2
[0037] The difference from Example 1 is that the yield of 2-amylanthraquinone is 16.1% by replacing the air flow rate of 40 mL / min with the oxygen flow rate of 40 mL / min.
Embodiment 3
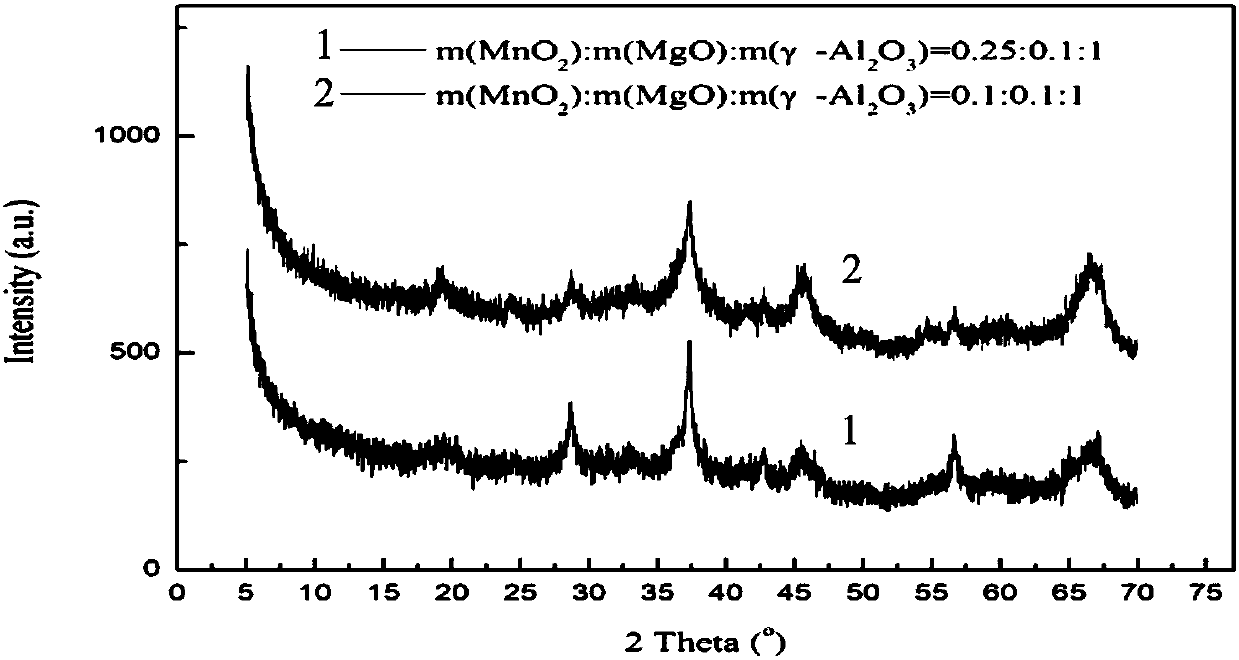
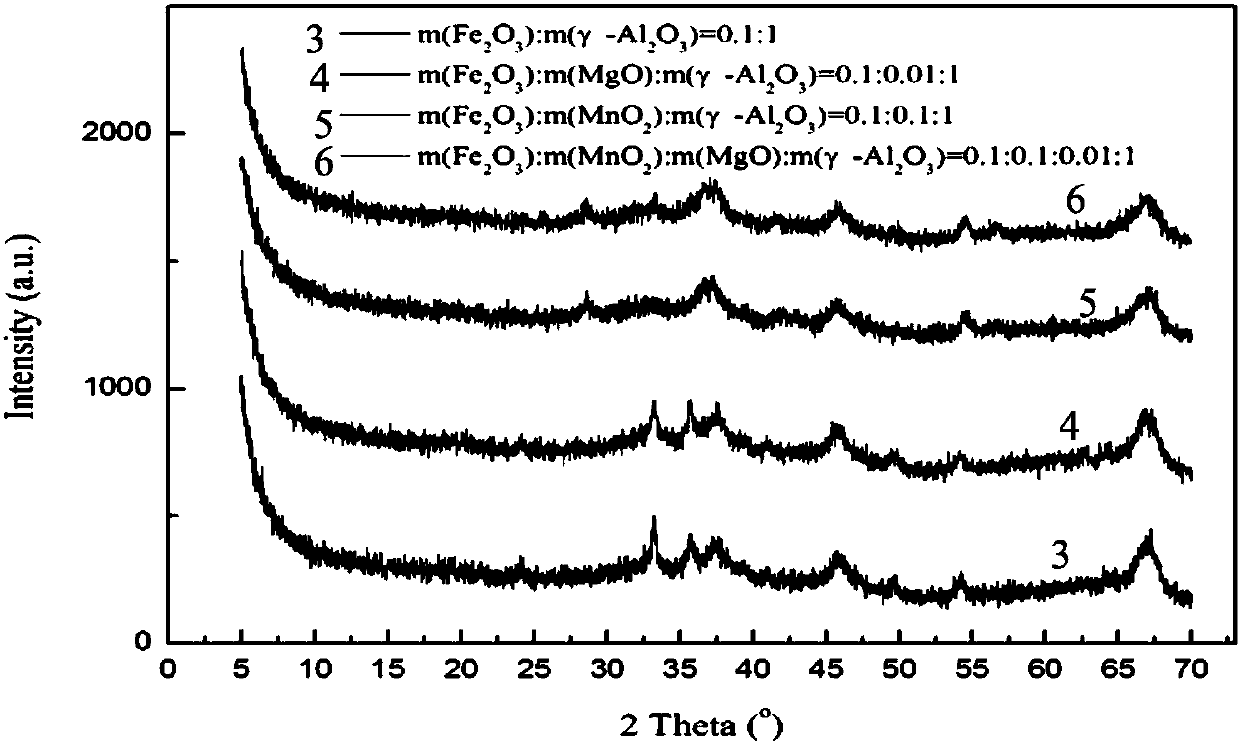
[0039] The difference with Example 1 is that the 0.25MnO 2 / 0.1MgO / γ-Al 2 o 3 Catalyst replaced with 0.25MnO 2 / γ-Al 2 o 3 , The yield of 2-amylanthraquinone was 15.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com