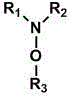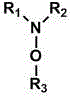Preparation method of p-benzoquinone compound
A technology of phenol compounds and compounds, which is applied in the field of preparation of p-benzoquinone compounds, and can solve the problems of excessive catalyst consumption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 250mL reactor, add cobalt nitrate hexahydrate (2.91g, 10.0mmol), N,N-diethylethoxyamine (1.18g, 10mmol), phenol (9.4g, 100mmol) and acetonitrile (78.5g, 100mL ), after mixing evenly, close the reactor and fill it with 30atm oxygen, stir and heat to 60 o C, after reacting for 3 hours, cool to room temperature, take out the mixture for analysis after degassing, and obtain remaining phenol: 5.4g, p-benzoquinone: 3.8g.
Embodiment 2-9
[0030] project Main catalyst (g, mmol) Phenol (g) p-Benzoquinone (g) 1. -- 9.2 0 2. Vanadium pentoxide (1.82, 10) 5.7 2.6 3. Manganese sulfate monohydrate (1.69, 10) 4.4 4.8 4. Iron Phthalocyanine (5.68, 10) 5.8 3.4 5. Copper iodide (1.9, 10) 6.9 2.3 6. Ruthenium trichloride (2.1, 10) 3.5 5.7 7. Rhodium trichloride hydrate (2.1, 10) 5.7 2.4 8. Potassium chloroplatinate (4.86, 10) 5.0 4.0
Embodiment 10-18
[0032] project Cocatalyst (g, mmol) Phenol (g) p-Benzoquinone (g) 1. -- 8.4 0.9 2. N,N-Dimethylmethoxylamine (0.75, 10) 6.0 3.1 3. N,N-Dipropylpropoxylamine (1.59, 10) 7.3 2.0 4. N,N-Diisopropylisopropoxyamine (1.59, 10) 3.1 6.0 5. N,N-dioctyloctyloxyamine (3.7, 10) 6.5 2.7 6. Hydroxylamine (0.33, 10) 6.9 2.1 7. 2,2,6,6-Tetramethylpiperidine-N-hydroxyl (1.6, 10) 8.4 0.9 8. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-N-hydroxyl (1.73, 10) 8.9 0.3 9. N,N-Diphenylmethoxylamine (2.0, 10) 5.1 4.1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com